Drug composite containing troxerutin and ADP receptor antagonist
A receptor antagonist and composition technology, applied in the field of new pharmaceutical compositions, can solve the problems such as no troxerutin reported in the literature, and achieve inhibition of platelet aggregation, reduction of bleeding rate, good anti-platelet activity and anti-platelet effect. The effect of suppository activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
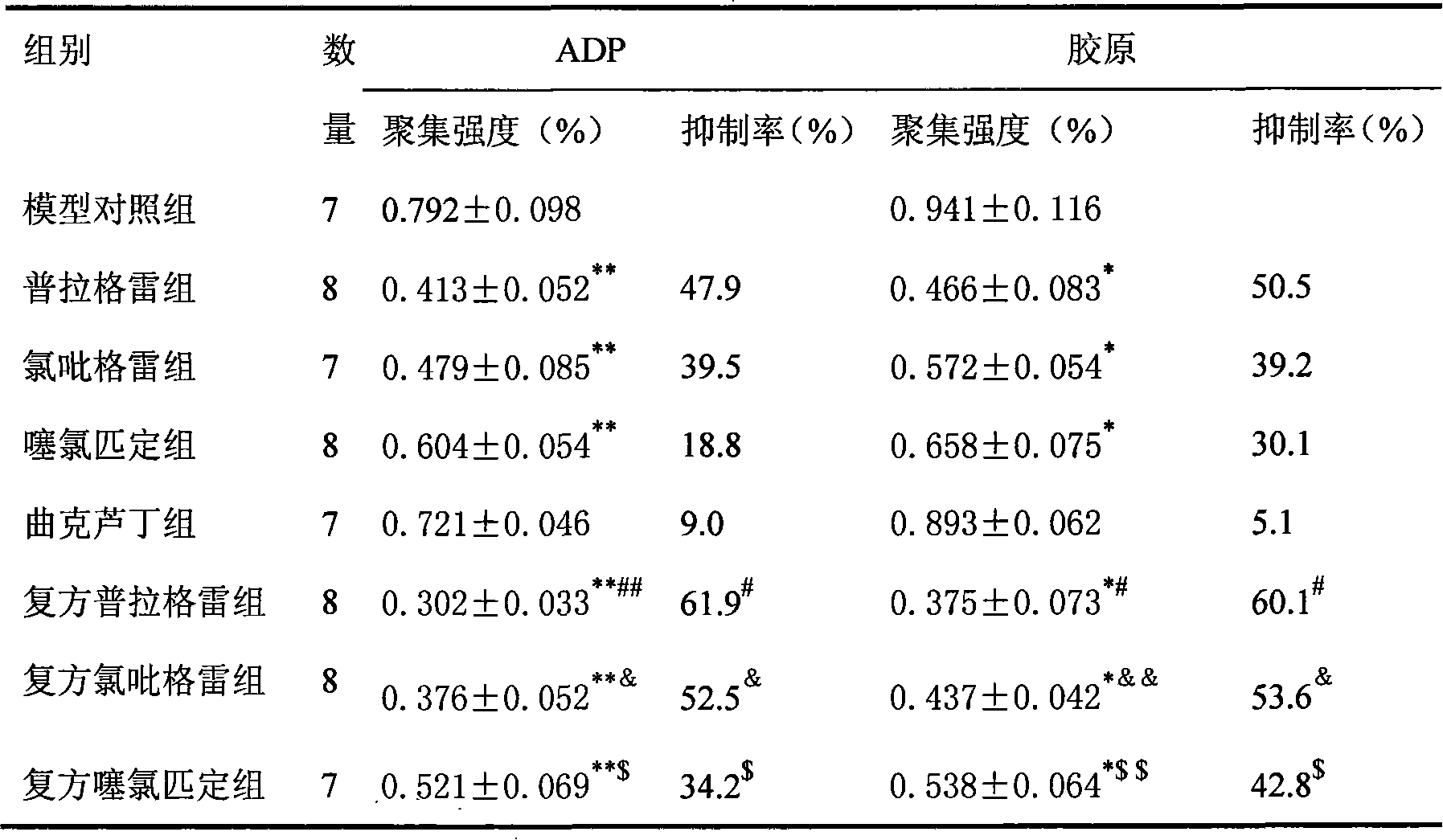
Embodiment 1
[0014] Embodiment 1 common tablet
[0015] Troxerutin 180g
[0016] Prasugrel Maleate 2g
[0017] Microcrystalline Cellulose 500g
[0018] Lactose 40g
[0019] 10% starch slurry appropriate amount
[0021] Preparation Process:
[0022] Troxerutin, prasugrel maleate, microcrystalline cellulose, and lactose were weighed and mixed uniformly. In addition, add an appropriate amount of 10% starch slurry to the mixed powder, mix evenly, make soft material, pass through a 18-mesh nylon sieve to make wet granules, and dry at about 60°C. The moisture content of the dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
Embodiment 2
[0023] Embodiment 2 common tablet
[0024] Troxerutin 60g
[0025] Clopidogrel Sulfate 5g
[0026] Starch 140g
[0027] Dextrin 120g
[0028] 50% ethanol appropriate amount
[0029] Magnesium Stearate 1.0g
[0030] Preparation Process:
[0031]Troxerutin, clopidogrel sulfate, starch and dextrin were weighed and mixed uniformly. In addition, an appropriate amount of 50% ethanol is added to the mixed powder, mixed evenly, made into soft material, passed through a 18-mesh nylon sieve to make wet granules, dried at about 60°C, and the moisture content of the dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
Embodiment 3
[0032] Embodiment 3 common tablet
[0033] Troxerutin 200g
[0034] Ticlopidine hydrochloride 50g
[0035] Microcrystalline Cellulose 500g
[0036] Lactose 40g
[0037] 10% starch slurry appropriate amount
[0038] Magnesium stearate 8g
[0039] Preparation Process:
[0040] Troxerutin, ticlopidine hydrochloride, microcrystalline cellulose and lactose were weighed and mixed uniformly. In addition, add an appropriate amount of 10% starch slurry to the mixed powder, mix evenly, make soft materials, pass through a 18-mesh nylon sieve to make wet granules, and dry at about 60°C. The moisture content of dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com