Seminal plasma micro ribonucleic acid marker related to spermatogenesis deficiency and application thereof
A technology of sperm production and markers, which is applied in the fields of genetic engineering and reproductive medicine, can solve the problems that the early diagnosis of diseases cannot meet the needs, the quality of individual semen fluctuates greatly, and it is not easy to monitor dynamically, so as to delay and prevent the progress of diseases and improve Sensitivity and specificity, effects of avoiding invasive diagnostics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1 Research Object Selection and Grouping Basis
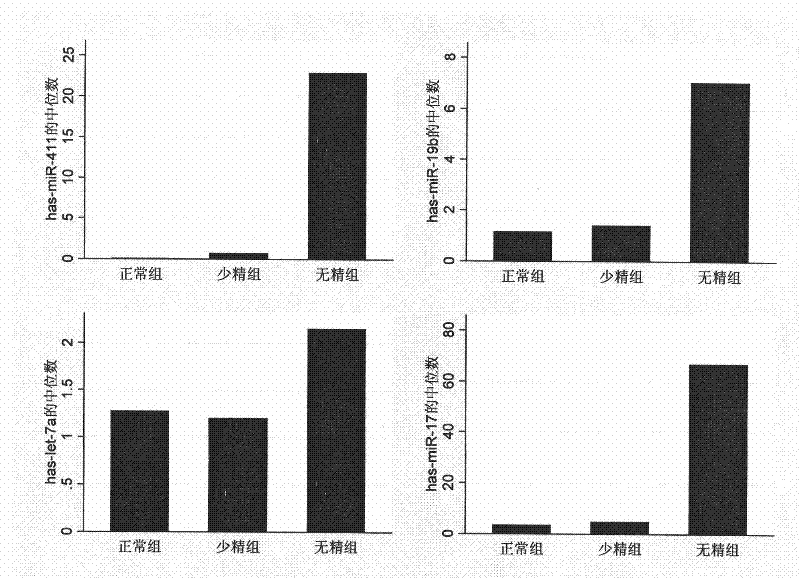
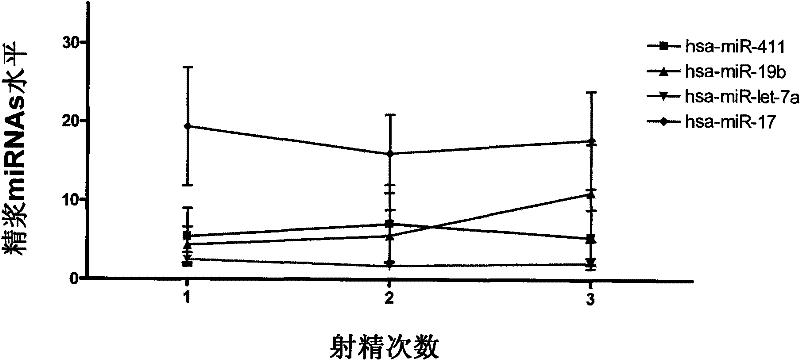
[0075] The inventor collected semen samples of adult males who met the requirements from the First Affiliated Hospital of Nanjing Medical University and the Nanjing Maternal and Child Health Hospital affiliated to Nanjing Medical University from September 2006 to September 2008. A total of 96 healthy fertile male controls (average age: 29.32±3.13), 96 cases of oligospermia patients (average age: 28.96±4.32), and 96 cases of azoospermia patients (average age: 28.76±4.07) were selected as Real-time PCR detection of miRNA expression experimental subjects ( figure 1 ). Specific sample classification criteria are as follows:
[0076] Group A: healthy fertile male control group (n=96)
[0077] 1. Between 24 and 34 years old
[0078] 2. No reproductive and endocrine system diseases
[0079] 3. No other systemic diseases
[0080] 4. Normal sexual function
[0081] 5. Normal semen quality
[0082] 6. Have a norm...
Embodiment 2
[0098] Example 2 Research object semen collection and routine analysis of semen quality
[0099]After at least 2 days of abstinence, subjects were asked to masturbate semen in a sterile wide-mouth plastic container in the room. After the semen samples were incubated at 37°C for about 30 minutes to liquefy, we performed routine semen analysis according to the WHO Human Semen Analysis Laboratory Manual (World Health Organization, 1999), including semen volume, sperm concentration, total number of ejaculates, sperm motility, Sperm motility and forward sex parameters, etc., mainly use μ-cell plate and computer-aided semen analysis system (CASA, WLJY 9000, Weili New Century Science & Tech Dev.). The rate of motile sperm was WHO standard "A" grade sperm (fast moving, velocity ≥ 25 μm / sec at 37°C) plus "B" grade sperm (slow advancing, velocity between 5 μm / sec and 25 μm / sec), while Grade "C" sperm (not advancing, velocity 6 / ml), the total number of one ejaculate (40×10 6 ) and spe...
Embodiment 3
[0100] Embodiment 3Real-time PCR method measures miRNA expression in seminal plasma
[0101] Primers (Table 1) were designed for quantitative Real-time PCR detection of miRNAs in the seminal plasma of 96 healthy fertile male controls, 96 oligospermia, and 96 azoospermia patients.
[0102] (1) Preparation of cDNA samples: a) Take 500 μl of seminal plasma; b) Add an equal volume of water-saturated phenol, shake and mix, centrifuge at 15,000 rpm at 4°C for 30 minutes, and take the supernatant; c) Add an equal volume of phenol to the supernatant Shake and mix with chloroform, centrifuge at 12,000 rpm for 30 minutes at 4°C, and take the supernatant; d) repeat steps b) and c) twice, and centrifuge at 12,000 rpm for 20 minutes. Take the supernatant as an RNA sample; e) Then obtain cDNA through RNA reverse transcription reaction. The reverse transcription reaction system includes 4 μl 5×AMV buffer, 2 μl 10 mM dNTP mixture (Takara), 0.5 μl RNase inhibitor (Takara), 1 μl AMV (Takara) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com