Novel method for protecting 31-position or 42-position hydroxyl of rapamycin by selective silicon etherification
A rapamycin, selective technology, applied in the field of pharmaceutical chemical synthesis, to achieve the effects of high production efficiency, easy operation and short reaction time
Inactive Publication Date: 2012-05-30
SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
But when repeating experiments, the inventors found that the method has very poor practical operability, the selectivity of the removal process is extremely low, only a small amount of target product can be obtained, and a long time low temperature reaction is required, which is difficult for practical application
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0036]
[0037] Example 2
Embodiment 2
[0039]
[0040] Example 3
Embodiment 3
[0042]
[0043] Example 4
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
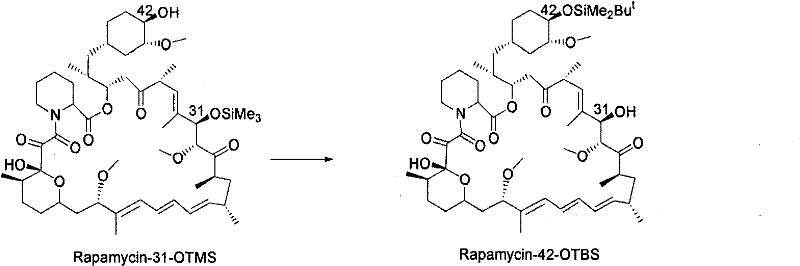
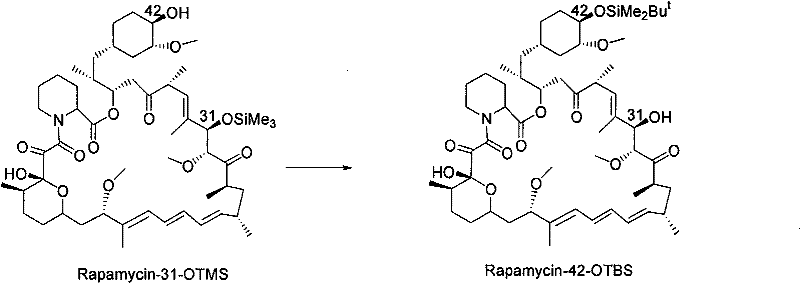
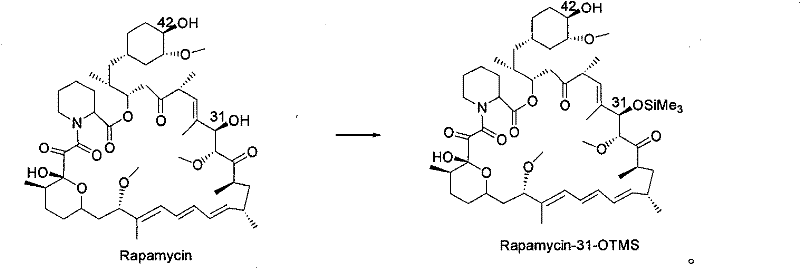
The invention discloses a novel method for protecting 31-position or 42-position hydroxyl of rapamycin by selective silicon etherification. The method comprises the following steps that: (1) the rapamycin reacts with chlorosilane R1, R2 and R3 SiCl in inert solvent in the presence of organic alkali, and a 31-position monoprotection product I of the rapamycin is obtained, wherein the R1, R2 and R3 are independently hydrogen, chain-shaped alkyl of between C1 and C6, phenyl, phenmethyl or p-xylyl respectively, but cannot be the hydrogen at the same time; and (2) when the R1, R2 and R3 in the step (1) are the same and are methyl, ethyl or isopropyl, the 31-position monoprotection product I of the rapamycin which is obtained in the step (1) reacts with tertiary butyl diphenyl chlorosilane in the inert solvent in the presence of the organic alkali, siloxane protecting group with poor 31-position stability is selectively removed, and a 42-position monoprotection product II is obtained. Compared with the prior synthesis method, the method has the characteristics of mild condition, simple and convenient operation, high region selectivity, high production efficiency and the like, and is suitable for production and preparation on a large scale.
Description
technical field [0001] The invention belongs to the field of pharmaceutical chemical synthesis, and relates to a new method for protecting the 31-position or 42-position hydroxyl of rapamycin through a selective silicon etherification reaction, more specifically, relates to a method using an organic base and a silane reagent in an inert solvent A new method for selectively protecting the 31- or 42-hydroxyl group of rapamycin in one pot. Background technique [0002] Rapamycin (RPM for short, trade name sirolimus) is a macrolide antibiotic extracted from the fermented liquid of Streptomyces hygroscopicus, and it has been used for anti-immune rejection after organ transplantation since 1989. In-depth studies have shown that rapamycin, as an mTOR inhibitor, also has significant anti-tumor activity. Through the modification of the 42-position hydroxyl group of rapamycin, many analogues that have been marketed or have market prospects have been discovered one after another, for...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07F7/18C07D498/18
CPCY02P20/55
Inventor 南发俊余琳千张仰明
Owner SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com