Method for preparing dithio-metal acid ammonium
A disulfide metal, metal technology, applied in non-metallic elements, chemical instruments and methods, sulfur compounds, etc., can solve the problems of complex preparation process, low product yield, long reaction time, etc., to achieve simple operation and reduce harm. , the effect of avoiding the influence of product purity and reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Pour 5g of industrial grade ammonium metatungstate (containing 83% tungsten trioxide) crystals into a conical beaker, then slowly add 35ml of 8wt% ammonium sulfide solution, at this time the color of the reaction solution will gradually change from blue to light yellow , pour all the ammonium sulfide solution into the beaker, stir at room temperature for 30 minutes, and finally let the reaction solution stand for 12 hours to obtain yellow crystals, filter and wash with deionized water and absolute ethanol three times respectively, vacuum at room temperature After drying, the final ammonium dithiotungstate crystal is obtained, and the yield is about 93% (calculated according to the tungsten content).
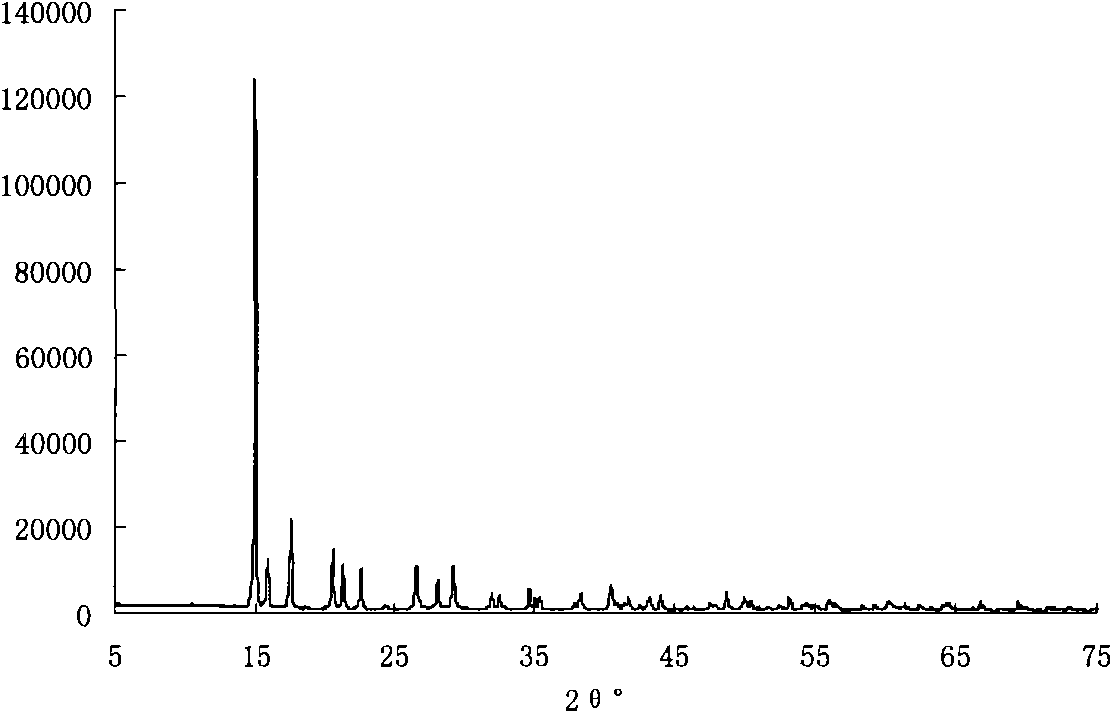
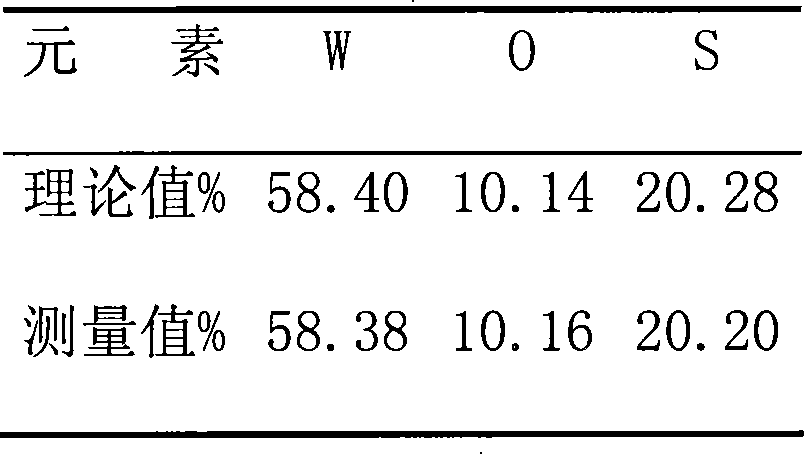
[0021] The XRD spectrogram of the product obtained in this embodiment is shown in figure 1 , XRF elemental analysis results are shown in Table 1.
[0022] From figure 1 It can be seen that the XRD spectrum of the product is completely consistent with the standard spectru...
Embodiment 2
[0026] Pour 8.3g of tungsten trioxide crystals into a conical beaker, and then slowly add 56ml of 10wt% ammonium sulfide solution. At this time, put the reaction solution in a water bath and heat up to 60°C. At this time, the color of the reaction solution will gradually change from blue to blue. After stirring for 10 minutes, the reaction solution was left to stand for 12 hours to obtain yellow crystals, which were filtered and washed three times with deionized water and absolute ethanol respectively, and vacuum-dried at room temperature to obtain the final thiooxytungstic acid Ammonium crystals, the yield is about 93% (calculated based on tungsten content).
Embodiment 3
[0028] Pour 12.36g of industrial-grade ammonium molybdate crystals into a conical beaker, and then slowly add 60ml of 8wt% ammonium sulfide solution. At this time, the color of the reaction solution will gradually change from blue to dark red. Pour all the ammonium sulfide solution into After being placed in the beaker, stir at room temperature for 30 minutes, and finally let the reaction solution stand for 12 hours to obtain blood-red crystals. After filtering, wash with deionized water and absolute ethanol three times respectively, and vacuum-dry at room temperature to obtain the final thioxo Ammonium molybdate crystals, the yield is about 93% (calculated based on molybdenum content).
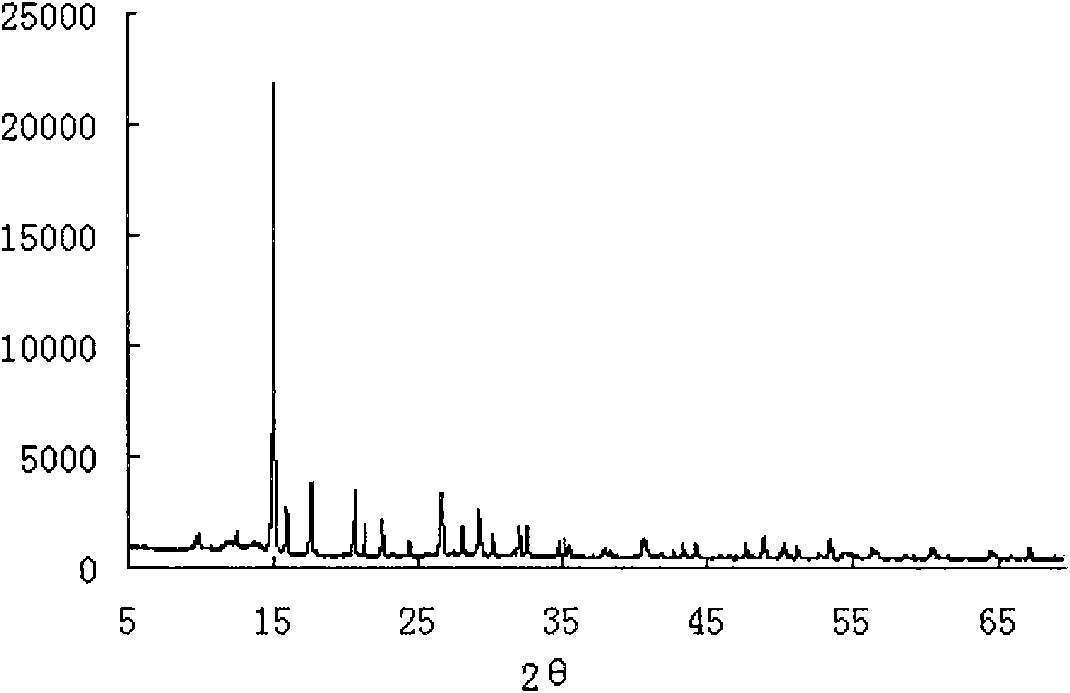
[0029] The XRD spectrogram of the product obtained in this embodiment is shown in figure 2 , XRF elemental analysis results are shown in Table 2.
[0030] figure 2 It can be seen that the XRD spectrum of the product is completely consistent with the standard spectrum, and each peak type i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com