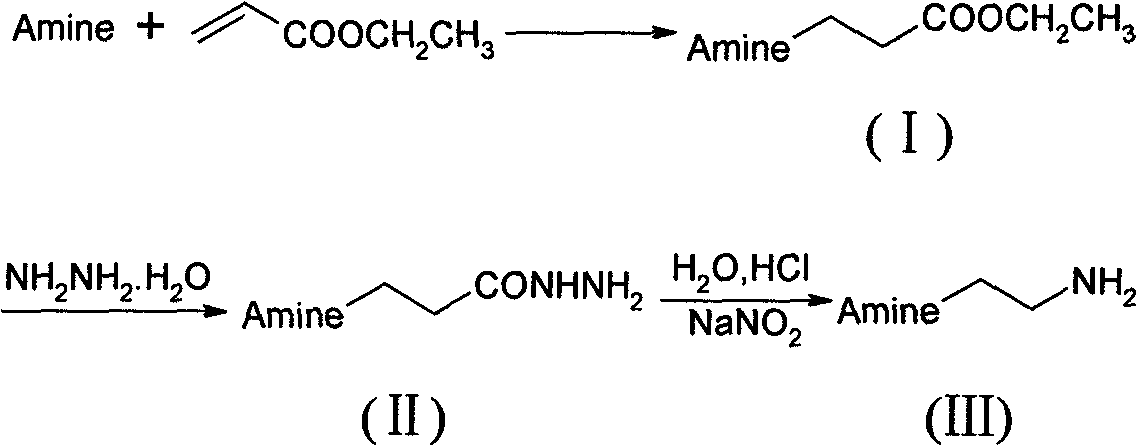
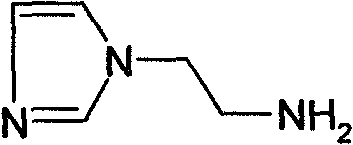Preparation method of N-substituted ethylene diamine derivative
A derivative, ethylenediamine technology, applied in the field of preparation of fine chemicals, can solve the problem of expensive 2-oxazolinone, and achieve the effects of high total yield, stable reaction and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] (1), the preparation of intermediate (I) a: add water (60ml), FeCl successively in 150ml there-necked flask 3 .6H 2 O (0.97g, 6mmol), imidazole (4.1g, 60mmol), ethyl acrylate (7.7ml, 72mmol) was slowly added dropwise, stirred, reacted at 30°C for 15h, column chromatography (CH 2 Cl 2 :CH 3 OH=40:1) isolated light yellow liquid compound (I)a (9.4g), yield 94.8%, 1 NMR (Cl 3 CD-d 6 )δ: 7.50(s, 1H), 7.04(s, 1H), 6.94(s, 1H), 4.26(t, 2H), 4.13(q, 2H), 2.77(t, 2H), 1.24(t, 3H ).
[0021] (2), the preparation of intermediate (II) a: 80% hydrazine hydrate (7.5ml, 150mmol), ethanol (30ml), slowly add dropwise ethanol (10ml) and intermediate (I) a (8.40g, 50mmol) The temperature of the mixture was not higher than 40°C. After stirring for about 30 minutes, the temperature was raised to reflux for 8 hours. Column chromatography (CH 2 Cl 2 :CH 3 OH=20:3) to obtain yellow compound (II)a (7.26g) with a yield of 94.3%. 1 HNMR (Cl 3 CD-d 6 )δ: 8.41(s, 1H), 7.52...
Embodiment 2
[0024]
[0025] According to the method for Example 1, benzylamine was used instead of imidazole to prepare (III)b, with a total yield of 72.1% (calculated as ethyl acrylate), 1 HNMR (Cl 3 CD-d 6 ) δ: 7.30 (m, 5H), 3.79 (t, 2H), 2.74 (m, 2H), 1.46 (m, 2H).
Embodiment 3
[0027]
[0028] According to the method for Example 1, (III)c was prepared with aniline instead of imidazole, with a total yield of 60.4% (calculated as ethyl acrylate), 1 HNMR (Cl 3 CD-d 6 )δ: 7.14(m, 2H), 6.67(m, 2H), 6.58(m, 1H), 4.10(s, 1H), 3.07(m, 2H), 2.82(m, 2H), 1.30(s, 2H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com