Methods of using mek inhibitors
A medicinal salt and patient technology, applied to medical preparations containing active ingredients, pharmaceutical formulas, anti-tumor drugs, etc., can solve the problem of reducing the transformation ability of mutant Ras
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0175] In one embodiment, cancer is at least partially regulated by inhibiting MEK.
[0176] In another embodiment, the cancer is selected from the group consisting of melanoma, colon cancer, rectal cancer, pancreatic cancer, breast cancer, non-small cell lung cancer, small cell lung cancer, papillary thyroid cancer, anaplastic thyroid cancer, endometrial cancer, and ovarian cancer.
[0177] In another embodiment, the one or more treatments are one or more chemotherapeutic agents.
[0178] In another embodiment, the one or more chemotherapeutic agents are selected from the group consisting of taxanes, platinum agents, topoisomerase inhibitors, alkylating agents, antimetabolites, antimicrotubule agents, and bcr-abl inhibitors . In another embodiment, the one or more chemotherapeutic agents are anti-microtubule agents selected from vincristine, vinblastine, vinorelbine, and vindesine.
[0179] In another embodiment, the one or more chemotherapeutic agents are selected from ra...
Embodiment 1
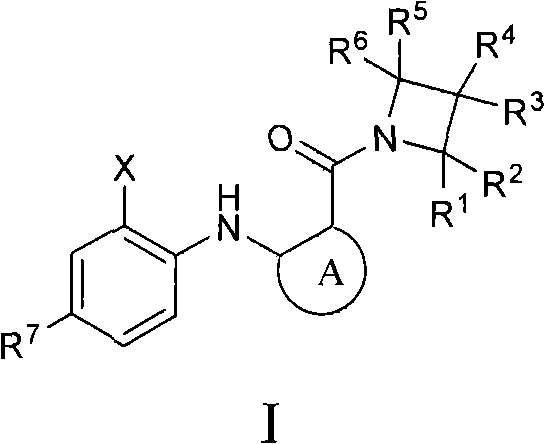
[1043] 1-({3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]phenyl}carbonyl)azetidin-3-ol
[1044]
[1045] A method similar to that described in US 7,019,033 was used to prepare 3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]benzoic acid (2.1 g, 5.3 mmol), which was added to DMF ( 10 mL), followed by the addition of PyBOP (2.6 g, 5.3 mmol), and the mixture was stirred at room temperature over 15 minutes. Azetidin-3-ol hydrochloride (870 mg, 8.0 mmol) and DIPEA (1.85 mL, 11.2 mmol) were then added and the mixture was allowed to continue stirring at room temperature for 1 hour. The mixture was then partitioned between ethyl acetate and 0.5M aqueous sodium hydroxide. The organic layer was then washed with water (3x), followed by brine, and dried over anhydrous sodium sulfate. Filtration and concentration followed by flash chromatography on silica gel using ethyl acetate:ethane (5:1) afforded 1-({3,4-difluoro-2-[(2- Fluoro-4-iodophenyl)amino]phenyl}carbonyl)azetidin-3-ol (2.09 g,...
Embodiment 2
[1053] N-[1-({3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]phenyl}carbonyl)azetidin-3-yl]-N2,N2-two Ethyl Glutamine
[1054]
[1055] 3,4-Difluoro-2-[(2-fluoro-4-iodophenyl)amino]benzoic acid (200 mg, 0.51 mmol) (prepared using a method similar to that described in US 7,019,033), PyBOP (256 mg, 0.51 mmol ), commercially available tert-butyl azetidin-3-ylcarbamate (131 mg, 0.77 mmol) and N,N-diisopropylethylamine (180 μL, 1.02 mmol) in dimethylformamide (3 mL) The solution in was stirred at room temperature for 15 hours. The reaction mixture was partitioned between 5% aqueous lithium chloride and ethyl acetate. The organic portion was washed with 20% aqueous citric acid, saturated aqueous sodium bicarbonate, brine, dried over sodium sulfate, filtered and concentrated in vacuo to give a brown residue which was purified by column chromatography on silica gel with 30% ethyl acetate / hexane Elution with alkane afforded [1-({3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]phenyl}c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com