Application of nattokinase in preparing cerebral protective agent type drugs
A technology of nattokinase and brain protection, which is applied in the direction of drug combination, medical formula, medical preparations containing active ingredients, etc., to achieve the effect of exact drug effect, small dosage and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 The therapeutic effect of nattokinase on focal cerebral ischemia in rats induced by photochemical method
[0035] 1.1 Test material
[0036] Nattokinase (abbreviated as DANK), white freeze-dried flocculent powder, provided by Chengdu Di'ao Pharmaceutical Group, batch number: 20071225, each 1.5g of DANK is equivalent to 200mg of pure product, after precise weighing, dissolve and dilute with normal saline for injection to the desired concentration.
[0037] Edaravone injection, colorless and clear liquid, produced by Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd., batch number: 80-071009, used as the original solution.
[0038] Wistar rats were provided by Shanghai Slack Experimental Animal Co., Ltd., animal certificate number: SCXK (Shanghai) 2003-0003. The animals were kept in a positive pressure purification and ventilation animal room with a room temperature of 23±2°C and a humidity of 40-70%. Artificial lighting simulated day and night changes, and f...
Embodiment 2
[0056] Example 2 Protective effect of DANK on ischemic injury of PC12 cells
[0057] 2.1 Test material
[0058] Cell line: PC12 rat adrenal pheochromocytoma clone, provided by the Cell Bank of Shanghai Chinese Academy of Sciences.
[0059] 2.2 Experimental model: PC12 cells were induced hypoxic injury by using sodium dithionite and low glucose medium.
[0060] 2.3 Index detection: MTT method, the OD value of each group was measured with an enzyme-linked immunosorbent assay at a wavelength of 570nm, and the cell viability was compared.
[0061] 2.4 Groups and results:
[0062] 2.4.1 Cell morphology observation
[0063] PC12 cell is a commonly used nerve cell line, the shape of the cell is fusiform and triangular, and the synapses are interwoven into a network with strong refractive index. After hypoxia treatment, the protrusions of the cells decreased or disappeared, the cells swelled and became round, the refractive index decreased, and some cells were lysed into fragments...
Embodiment 3
[0070] The preparation of embodiment 3 nattokinase injection
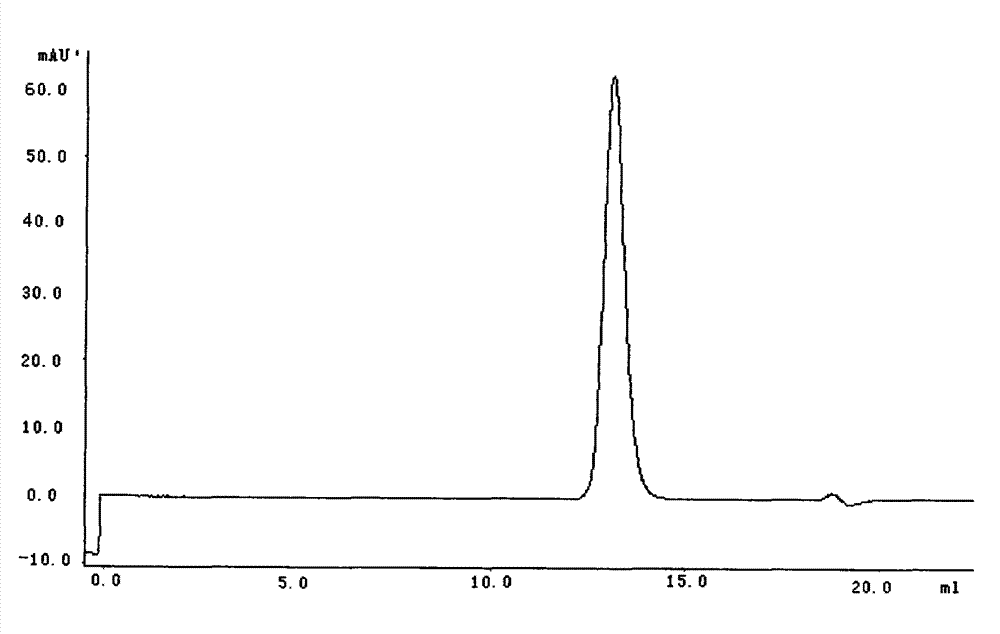
[0071] Get purified nattokinase solution (5mg / ml) 100ml, add 4% sucrose, 4% glucose and 5% mannitol. After dissolving, filter and sterilize, pack according to 2ml per bottle, aseptically freeze-dry and seal. Use physiological saline as solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com