High-efficiency purification process for acid leaching solutions of active substances in waste lithium batteries
A waste lithium battery and acid leaching solution technology, which is applied in the direction of improving process efficiency, can solve the problems of high cost, large loss of cobalt in hydrolysis precipitation, and difficult regeneration of extraction agent, etc., and achieve the effect of large flexible operation and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
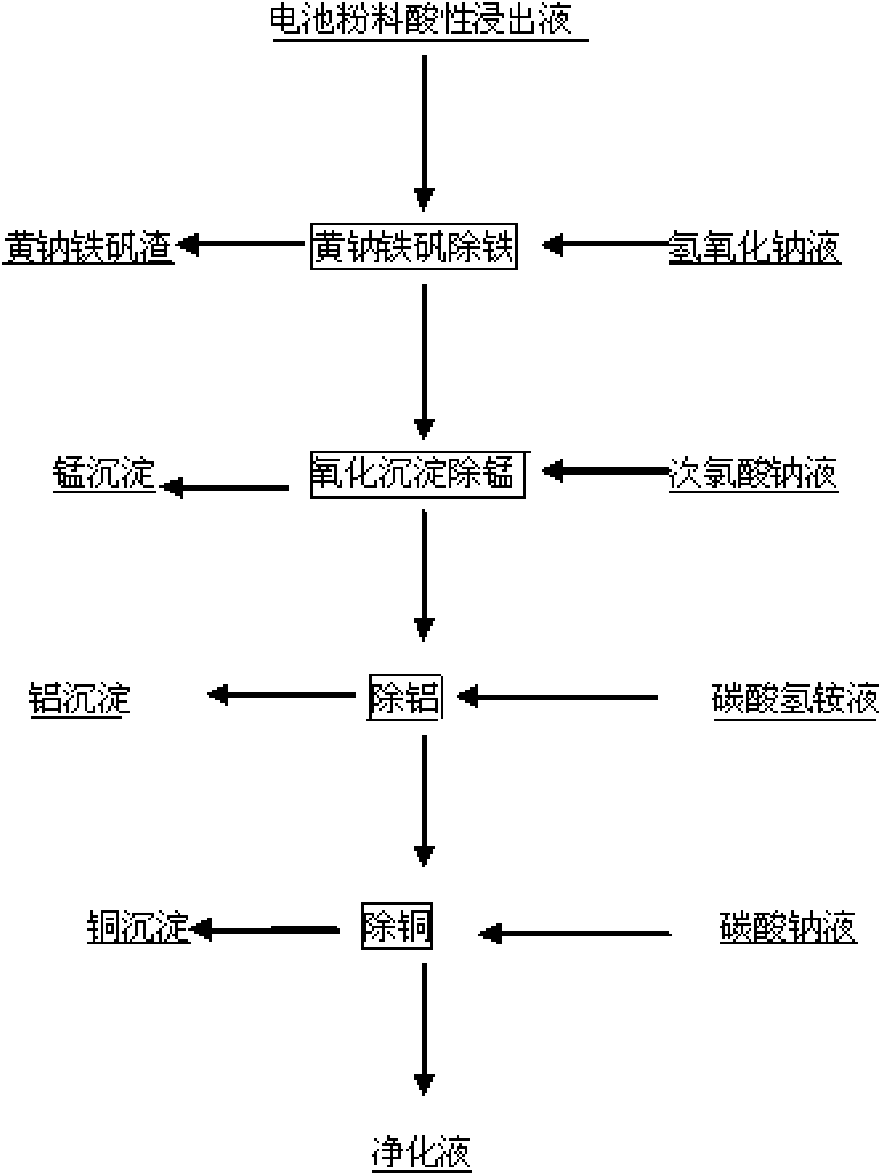
[0019] Measure 500ml of the acidic leaching solution of the positive electrode active material of the waste lithium battery, which contains metal ions such as cobalt, copper, aluminum, manganese, iron, nickel, etc., and the initial pH value is 0.4. After the temperature rises to 90°C, add 500g / L to the solution NaOH solution to adjust the pH value to 1.0, then slowly add NaOH solution with a mass percentage concentration of 10% to adjust the pH value to 1.5, stir for 2 hours, then control the end point pH value to 2.0, filter, and slowly add a theoretical amount of 110% mass to the filtrate The percentage concentration is 10% sodium hypochlorite solution, react at 50°C for 2 hours, use the NaOH solution with a mass percentage concentration of 5% to control the pH to about 2.5 during the reaction, filter, and slowly add a mass percentage concentration of 10% ammonium bicarbonate solution to the filtrate , adjust the pH value to 3.5 at 90°C, react for half an hour and filter, slo...
Embodiment 2
[0021] Measure 500ml of the acidic leaching solution of the positive electrode active material of the waste lithium battery, which contains metal ions such as cobalt, copper, aluminum, manganese, iron, nickel, etc., and the initial pH value is 0.5. After the temperature rises to 90°C, add 500g / L to the solution NaOH solution to adjust the pH value to 1.2, then slowly add NaOH solution with a mass percentage concentration of 10% to adjust the pH value to 1.7, stir for 2 hours, then control the end point pH value to 2.3, filter, and slowly add a theoretical amount of 110% by mass to the filtrate Fraction is 10% sodium hypochlorite solution, reacts at 70 DEG C for 2 hours, and the reaction process is controlled pH 2.5 with the NaOH solution of 5% mass percent concentration, filters, and slowly adding mass percent concentration in the filtrate is 10% mass percent concentration Ammonium bicarbonate solution, Adjust the pH value to 3.7 at 90°C, react for half an hour and filter, slow...
Embodiment 3
[0023]Measure 500ml of the acidic leaching solution of the positive electrode active material of the waste lithium battery, which contains metal ions such as cobalt, copper, aluminum, manganese, iron, nickel, etc., and the initial pH value is 0.6. After the temperature rises to 90°C, add 500g / L to the solution NaOH solution to adjust the pH value to 1.4, then slowly add NaOH solution with a mass percentage concentration of 10% to adjust the pH value to 1.7, stir for 3 hours, then adjust the final pH value to 2.3, filter, and slowly add a theoretical amount of 120% by mass to the filtrate Fraction is 10% sodium hypochlorite solution, reacts at 70 DEG C for 2 hours, and the reaction process is controlled pH 2.5 with the NaOH solution of 5% mass percent concentration, filters, and slowly adding mass percent concentration in the filtrate is 10% mass percent concentration Ammonium bicarbonate solution, Adjust the pH value to 4.0 at 90°C and react for half an hour to filter, slowly a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com