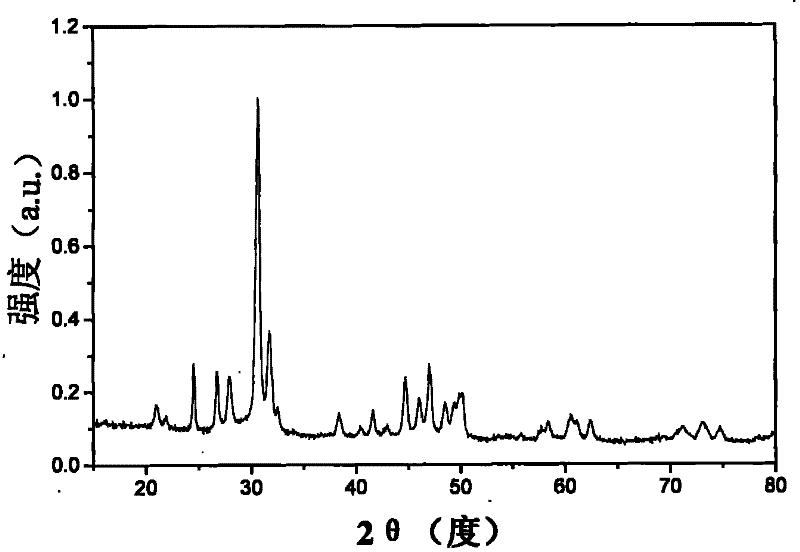
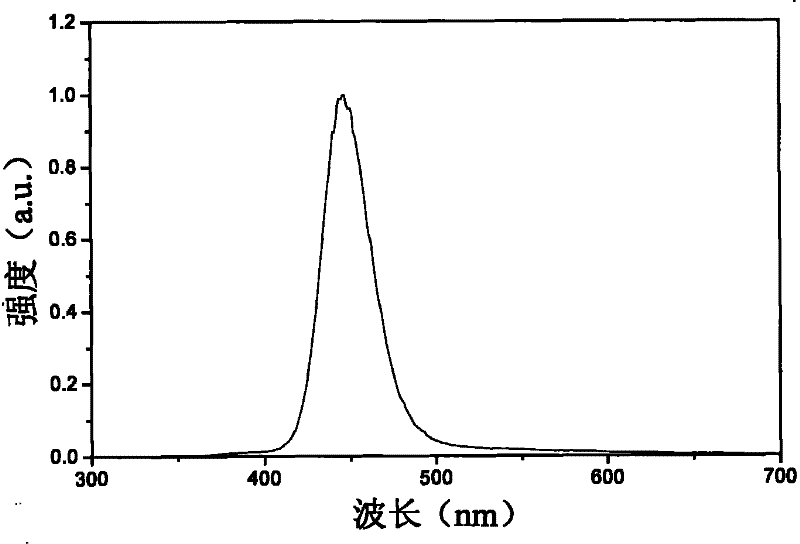
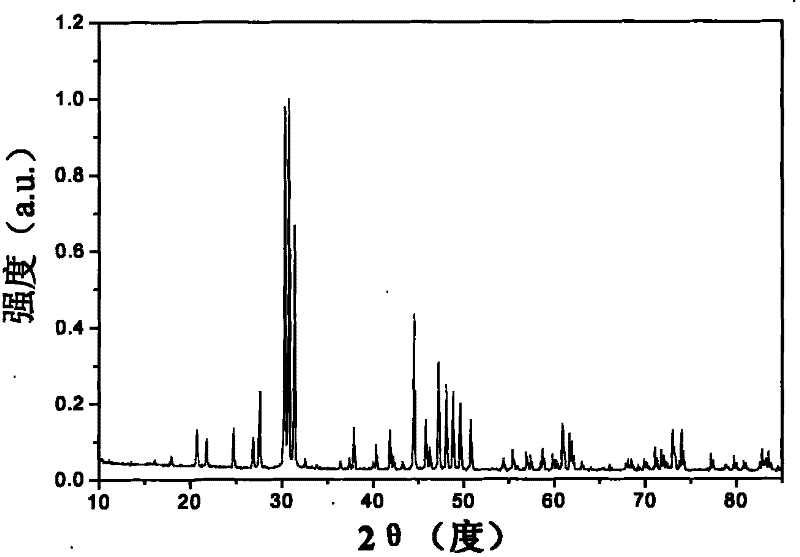
Hydroxide phosphate compound and method for preparing fluorophor by same
A technology of hydroxyphosphate compounds and phosphors, which is applied in the fields of phosphates, rare earth metal compounds, chemical instruments and methods, etc., and can solve the problems of easy deviation of the ratio of hydrogen phosphate and oxalate, impurity of the final product phase, and decrease of luminous intensity and other problems, to achieve the effect of reducing the decline of luminous performance, reducing the sintering temperature, and the ratio is easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh and prepare SrCl 2 , BaCl 2 , CaCl 2 , MgCl 2 and EuCl 3 Solution, the concentration of which is accurately calibrated is 2.5485mol / L, 1.008mol / L, 1.8528mol / L, 0.7562mol / L, 0.656mol / L. The dosage of each solution is shown in Table 1, mixed into solution A; weigh 22.37g (NH 4 ) 3 PO 4 , dissolved in 500ml of water, adjusted to pH 10.5 with ammonia water, and recorded as solution B.
[0038]Add solution A to solution B, and keep stirring. During the reaction, use ammonia water to control the pH value to 10.5, and the reaction time is 0.5h. After the reaction is completed, age the precipitate for 10h, filter, wash, and oven-dry at 110°C to obtain the precursor powder.
[0039] Adding the corresponding alkaline earth metal chlorides and 25% NH as shown in Table 1 is 5% by mass relative to the total amount of raw material in the precursor 4 Cl, ground and mixed evenly, and fired for 1 h in a reducing atmosphere of carbon powder at 1200 °C.
Embodiment 2~12
[0041] SrCl 2 , BaCl 2 , CaCl 2 , MgCl 2 and EuCl 3 The concentration of the solution is the same as in Example 1. The consumption of each embodiment solution is shown in Table 1, and is mixed into solution A; Weigh 19.81g (NH 4 ) 2 HPO 4 , dissolved in 500ml of water, adjusted to pH 10.7 with ammonia water, and recorded as solution B.
[0042] Add solution A to solution B, and keep stirring. During the reaction, use ammonia water to control the pH value to 10.7, and the reaction time is 1h. After the reaction is completed, age the precipitate for 12h, filter, wash, and dry in an oven at 110°C to obtain the precursor powder. .
[0043] Adding the corresponding alkaline earth metal chlorides and 6% NH as shown in Table 1 is 15% by mass relative to the total amount of raw material in the precursor 4 Cl, ground and mixed evenly, and fired for 1 h in a reducing atmosphere of carbon powder at 1000 °C. After washing with water and drying, the phosphors with the composition...
Embodiment 13~15
[0050] Weigh Sr(NO 3 ) 2 , Ba(NO 3 ) 2 , Ca(NO 3 ) 2 and Eu(NO 3 ) 3 And prepare the corresponding solution, and accurately calibrate the concentration of xxxxxxxxxxxxxxxxxx to be 2.4475mol / L, 1.105mol / L, 1.5684mol / L, and 0.478mol / L respectively. The dosage of each embodiment solution is shown in Table 3, and 5.23ml EuCl 3 The solutions were mixed into solution A; weighed 17.26g NH 4 h 2 PO 4 , dissolved in 500ml of water, adjusted to pH 11.4 with sodium hydroxide, and recorded as solution B.
[0051] Add solution B to solution A, and keep stirring. During the reaction, the pH value is controlled by sodium hydroxide to 11.4, and the reaction time is 0.5h. After the reaction is completed, the precipitate is aged for 16h, filtered, washed, and oven-dried at 110°C to obtain Precursor powder.
[0052] As shown in Table 3, adding the corresponding alkaline earth metal halide and 10% ammonium halide relative to the mass percentage of the total amount of raw materials int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com