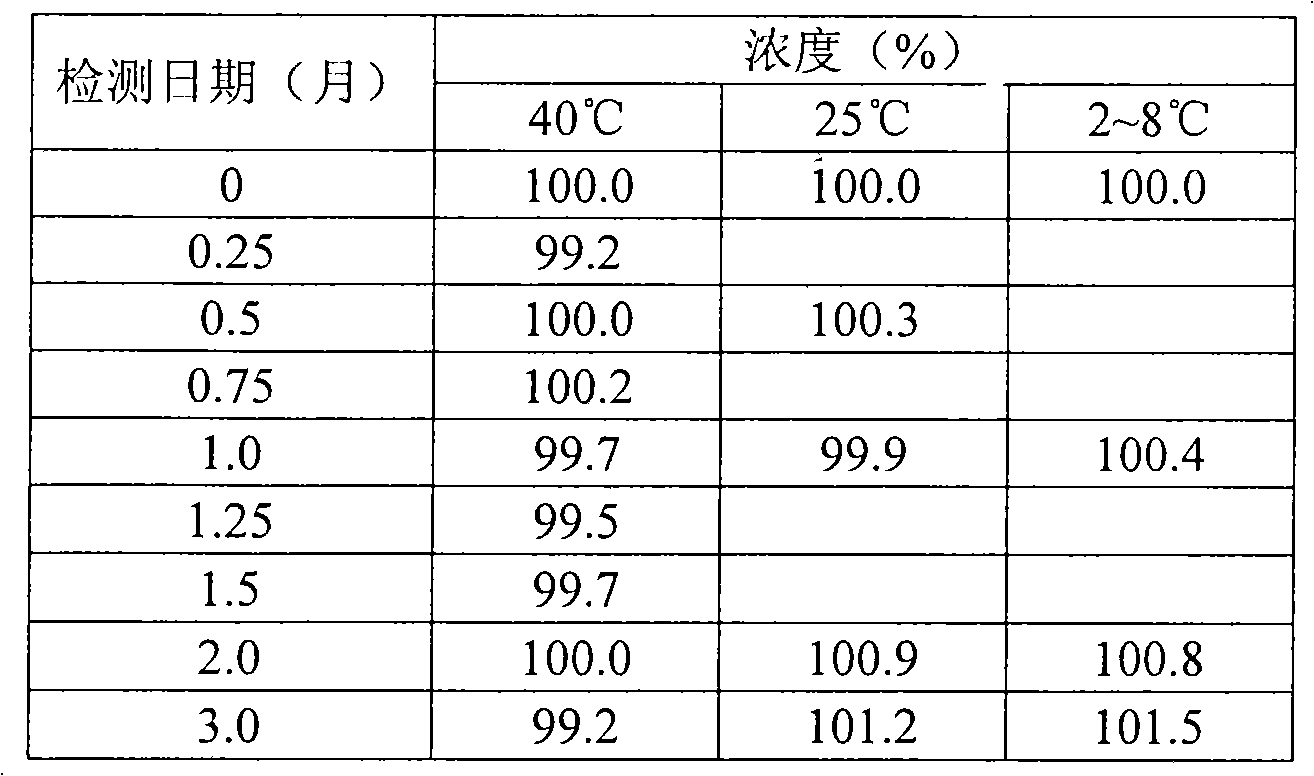
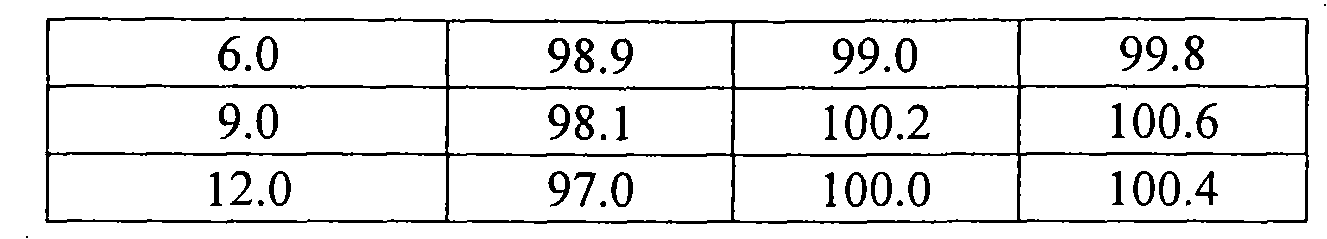
Propofol composition
A composition, propofol technology, applied in the field of propofol composition, can solve the problems of increasing product cost, great pain, easy to encourage microorganisms, etc., and achieve the effect of reducing production cost, reducing pain of injection, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0038] Preparation composition (10mL):
[0039] Propofol 50mg
[0040] Mannosyl-beta-cyclodextrin 1500mg
[0041] Bovine Serum Albumin 100mg
[0042] Acetylcysteine 0.5mg
[0043] Glycerin 1.8mg
[0044] Disodium hydrogen phosphate 85mg
[0045] Appropriate amount of NaOH or HCl
[0046] Appropriate amount of water
[0047] Preparation method: Dissolve disodium hydrogen phosphate in water for injection, adjust the pH to 8.5 (using HCl or NaOH) to prepare a buffer solution, add mannosyl-β-cyclodextrin, bovine serum albumin, glycerol, acetylcysteine Amino acid was added to the buffer solution, after it was completely dissolved, add propofol and stirred until the propofol was completely dissolved, then adjust the pH value to 8.5, then use the prepared disodium hydrogen phosphate buffer solution to make up the volume, filter and sterilize the solution and fill it with Packed in type I controlled vials to become the final product.
preparation Embodiment 2
[0049] Preparation composition (10mL):
[0050] Propofol 100mg
[0051] Hydroxypropyl-β-cyclodextrin 1500mg
[0052] Human Serum Albumin 300mg
[0053] ETDA 3.0mg
[0054] Sodium dihydrogen phosphate 60mg
[0055] Appropriate amount of NaOH or HCl
[0056] Appropriate amount of water
[0057] Preparation method: Dissolve sodium dihydrogen phosphate in water for injection, adjust the pH to 7.7 (using HCl or NaOH) to prepare a buffer solution, add hydroxypropyl-β-cyclodextrin, human serum albumin, and EDTA to the buffer solution, After complete dissolution, add propofol and stir until propofol is completely dissolved, adjust the pH value to 7.7, then use the prepared sodium dihydrogen phosphate buffer solution to make up the volume, filter and sterilize the solution and fill it into type I control vials Into the final product.
preparation Embodiment 3
[0059] Preparation composition (10mL):
[0060] Propofol 200mg
[0061] Hydroxypropyl-β-cyclodextrin 3000mg
[0062] Recombinant Albumin 1000mg
[0064] EDTA 5mg
[0065] Sodium citrate 192mg
[0066] Appropriate amount of NaOH or HCl
[0067] Appropriate amount of water
[0068] Preparation method: Dissolve sodium citrate in water for injection, adjust the pH to 7.5 (using HCl or NaOH) to prepare a buffer solution, add hydroxypropyl-β-cyclodextrin, recombinant albumin, sodium chloride, and EDTA to the buffer Solution, after completely dissolving, add propofol and stir until propofol is completely dissolved, adjust the pH value to 7.5, then use the prepared sodium citrate buffer solution to make up the volume, filter and sterilize the solution and fill it into type I control penicillin Bottled into the final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com