Drug residue competition-type quantum dot-labeled immunochromatography assay test-strip and observation device thereof
A technology of quantum dots and test strips, which is applied in the field of drug residue competitive quantum dot labeling immunochromatography detection card and its observation device, achieving the effects of high sensitivity, simple detection device and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] 3. Preparation of immunochromatographic detection card
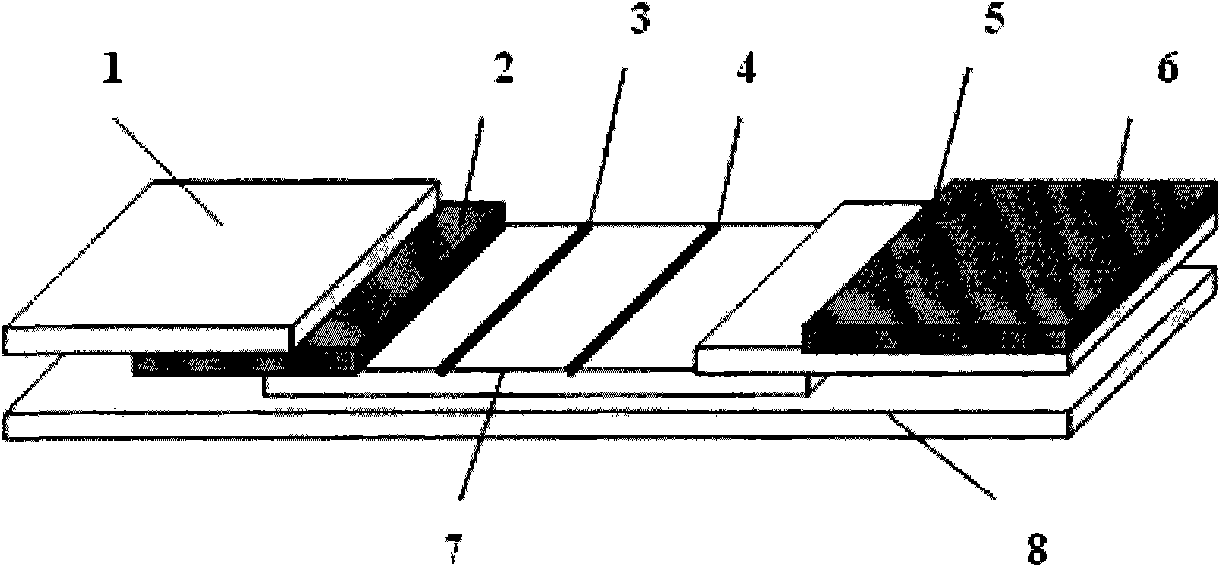
[0049] On a single-sided polyester or plastic plate, paste sample pad 1, glass fiber membrane 2 coated with quantum dot-labeled antibody, nitrocellulose membrane or cellulose acetate membrane coated with antibody in parallel, and water-absorbing pad 5 and pH Test paper, each layer must be closely connected, after pasting, cut into 3-5mm wide test paper strips, paste the precision pH test paper with a color change range of 5.5-9.0 on the absorbent paper, and then get the immunochromatography test strip 11 , assembled into the test card package after drying, and stored in the dark at 4-25°C.
[0050] 4. Sample testing and result judgment
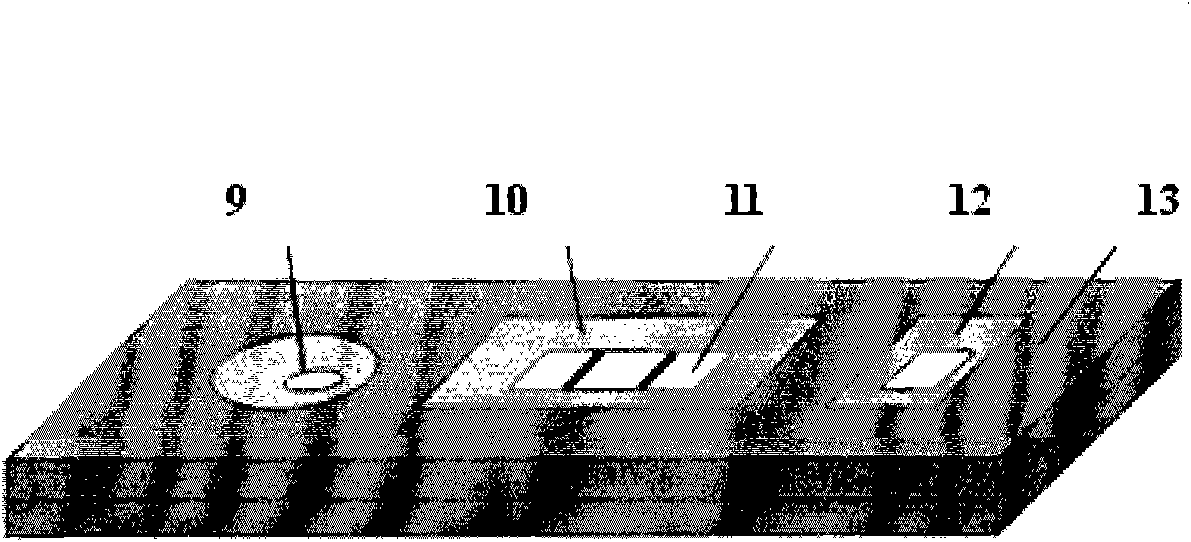
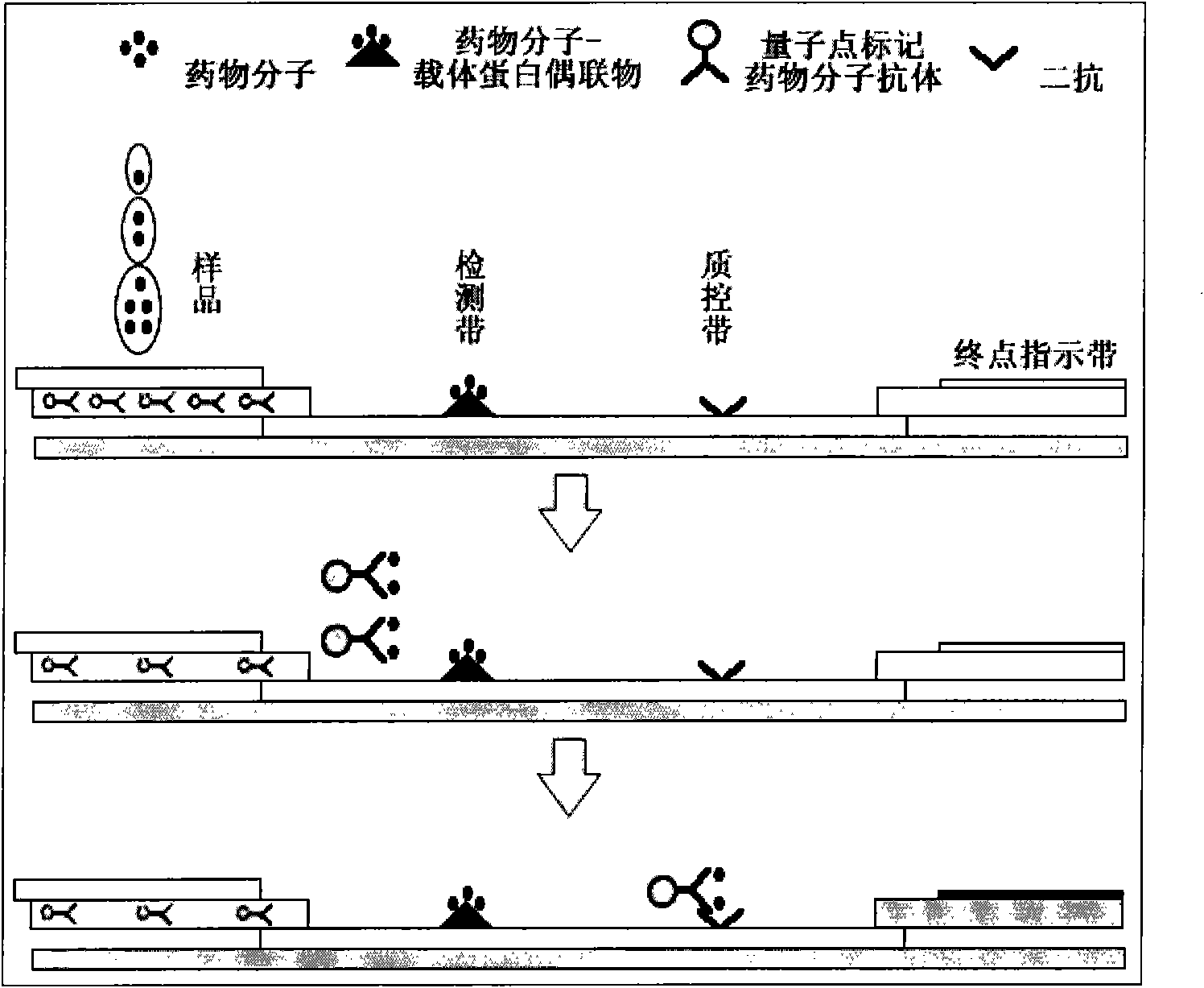
[0051] Use a dropper to drop the sample liquid to be tested in the sample tank, insert it into the slot 16 of the observation device after the color of the end point indicator window 13 changes, and observe the detection zone 3 and Fluorescence intensity changes of quality contr...
Embodiment 1
[0055] NaBH 4 Dissolve 0.5g and 1.68g of Te powder in 20ml of distilled water, react under nitrogen protection to form NaHTe, then adjust the pH value to 8.5 with concentrated ammonia water, add CdCl 2 2.5H 2O 1.5g, water bath 90°C, magnetic stirring, heating to reflux. Within 80 minutes of reaction time, samples of aqueous solution containing CdTe were taken out at regular intervals. The particle sizes of quantum dots obtained at different reaction times were different. After centrifugal sedimentation, they were washed with distilled water for 4 times to obtain CdTe quantum dots. CdTe was made into 0.5 mg / ml in water. Then, under the protection of Ar, the Zn precursor and the S precursor were slowly added to the CdTe quantum dot solution to a final concentration of 0.45 mg / ml and 0.16 mg / ml (freshly prepared 220 mg / ml zinc acetate and 1 mg / ml sulfurized Sodium aqueous solution), magnetic stirring, reflux in a water bath at 100°C, and after full reaction, yellow to pink co...
Embodiment 2
[0062] 1.2mL 0.15mol / L CdSO 4 Dilute with about 20mL of deionized water, inject 0.6mL of 2.0% trisodium citrate solution into this solution, and then add 0.75mL of 0.2mol / L Na 2 S was added to the mixture for reaction, and samples of the CdS solution were taken at regular intervals. The quantum dots obtained at different reaction times had different particle sizes, and were demulsified and washed with acetone, centrifuged, and the precipitate was collected to obtain the product CdS quantum dots. NaBH 4 0.5g and 0.5g of Se powder were dissolved in 20ml of distilled water, reacted to generate NaHSe under the protection of nitrogen, then adjusted the pH value to 8.8 with concentrated ammonia water, then mixed 20mmol / L ZnCl and 20mmol / L mercaptopropionic acid in equal volume ratio, adjusted When the pH value reaches 11, the NaHSe solution is injected to a concentration of 3 mmol / L to obtain a ZnSe solution. Then the CdS quantum dot solution was slowly added to the ZnSe solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com