Immunodiagnosis kit for detecting IV-type dengue virus NS1 antigen
A technology for dengue virus and immunodiagnosis, applied in the field of medicine, can solve the problems of reducing dengue virus, inability to distinguish four serotypes of dengue virus, etc., achieving high sensitivity, conducive to early diagnosis and treatment, high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
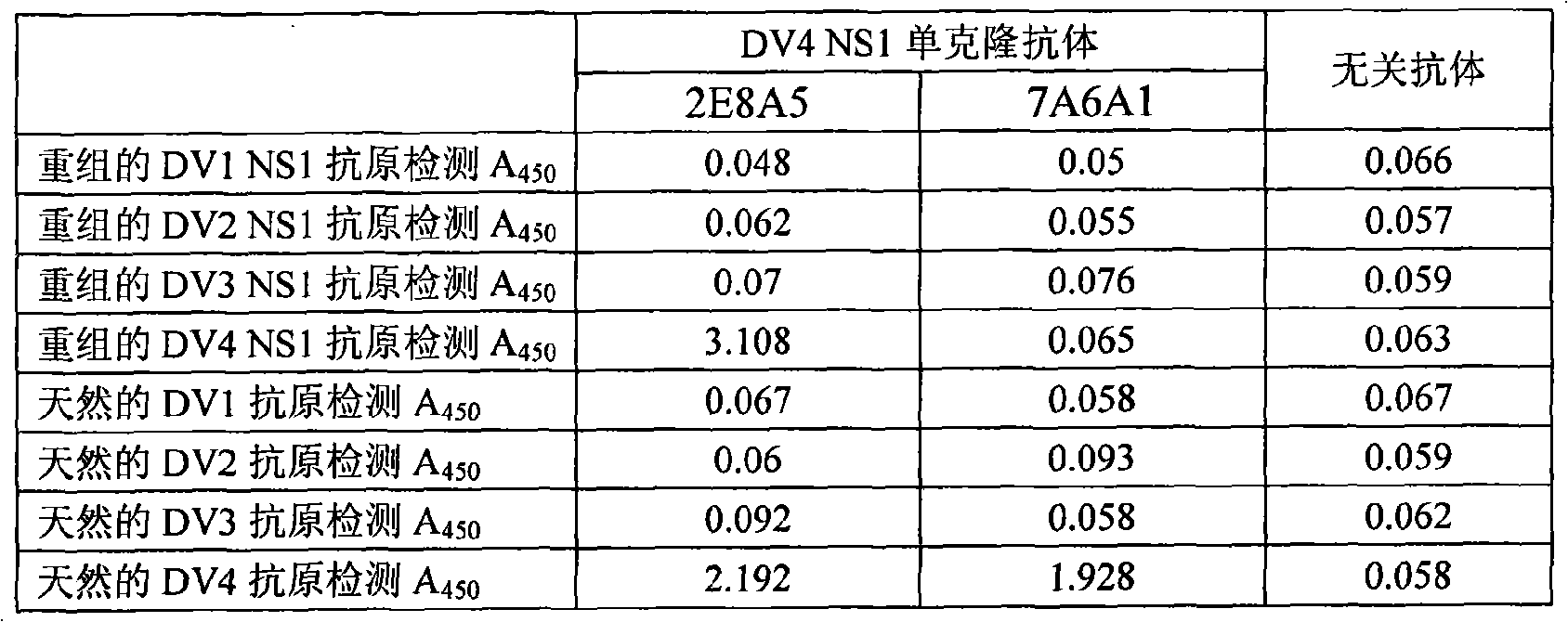
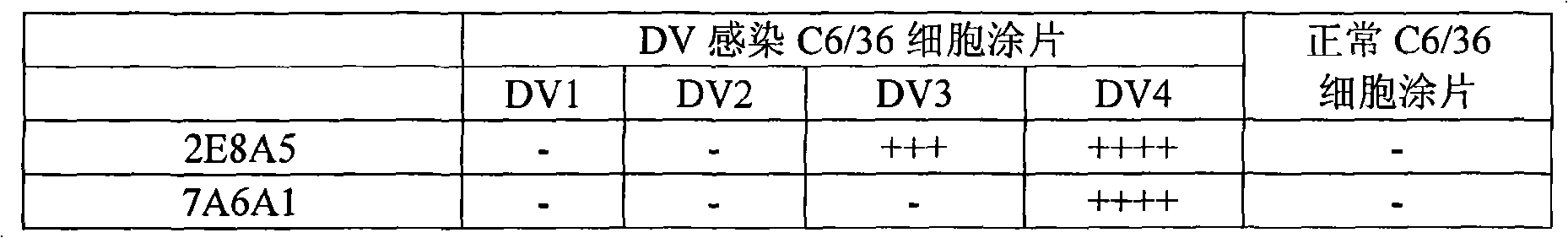
[0011] Below further illustrate kit of the present invention by concrete example and experimental report:
[0012] 1. The preparation method and identification results of the monoclonal antibodies 2E8A5 and 7A6A1:
[0013] (1) Preparation of immune antigen
[0014] The immunogen used to prepare the monoclonal antibody in the present invention is gene recombinant DV4NS1 protein and inactivated natural virus antigen. The gene recombinant DV4 NS1 protein is prepared by an engineering strain carrying the DV4 NS1 gene, and its preparation is carried out according to a conventional method, and the NS1 antigen is obtained by purifying with nickel primary nitrogen triacetate metal affinity chromatography, and the detailed preparation The method can refer to the user manual. After NS1 protein was purified, it was quantified with Coomassie protein assay reagent (PIERCE, Cat, No. ED62976). The results of Western blot identification of the purified recombinant protein showed that the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com