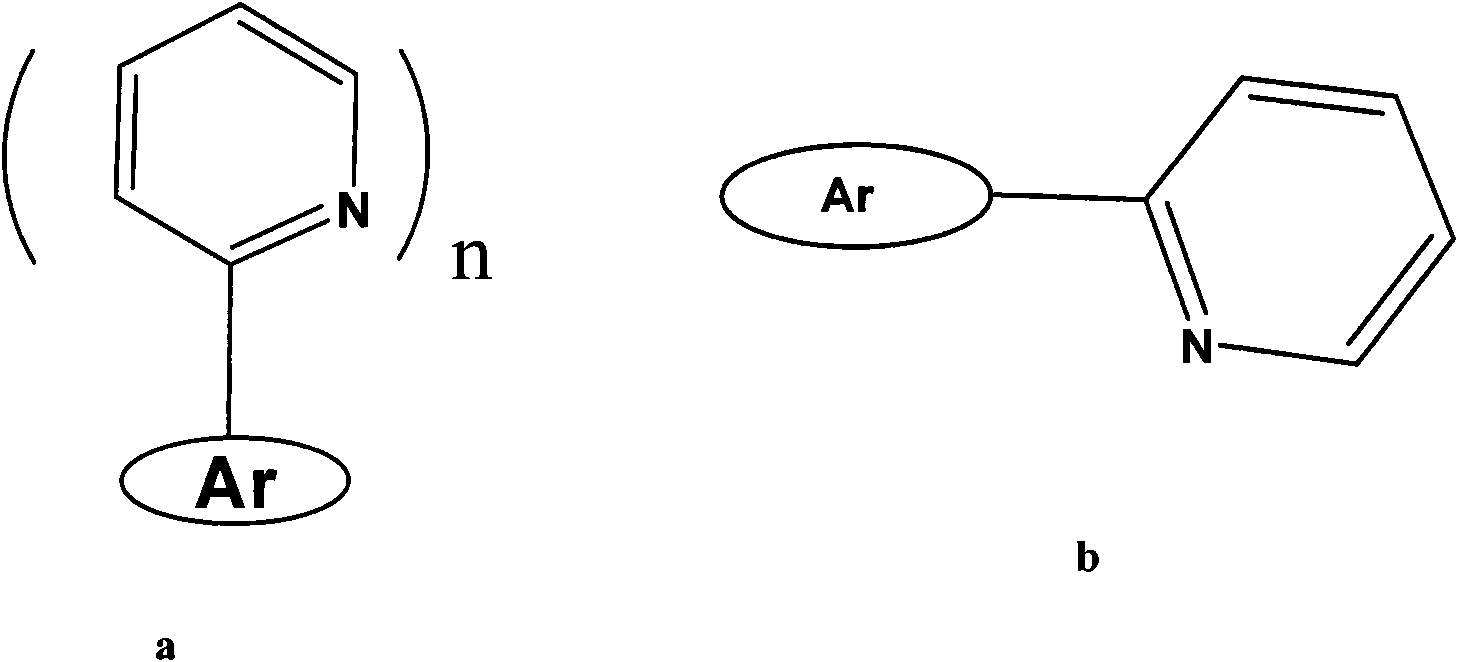
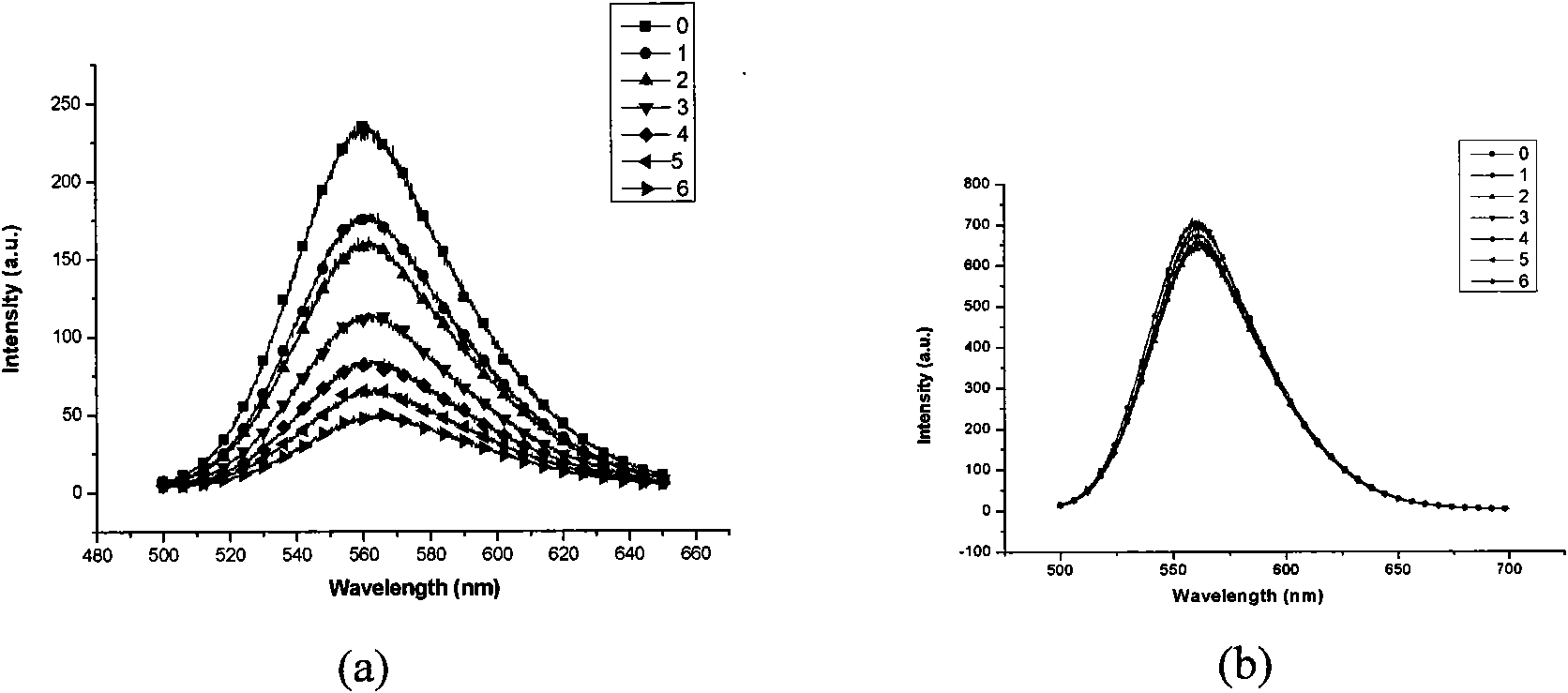
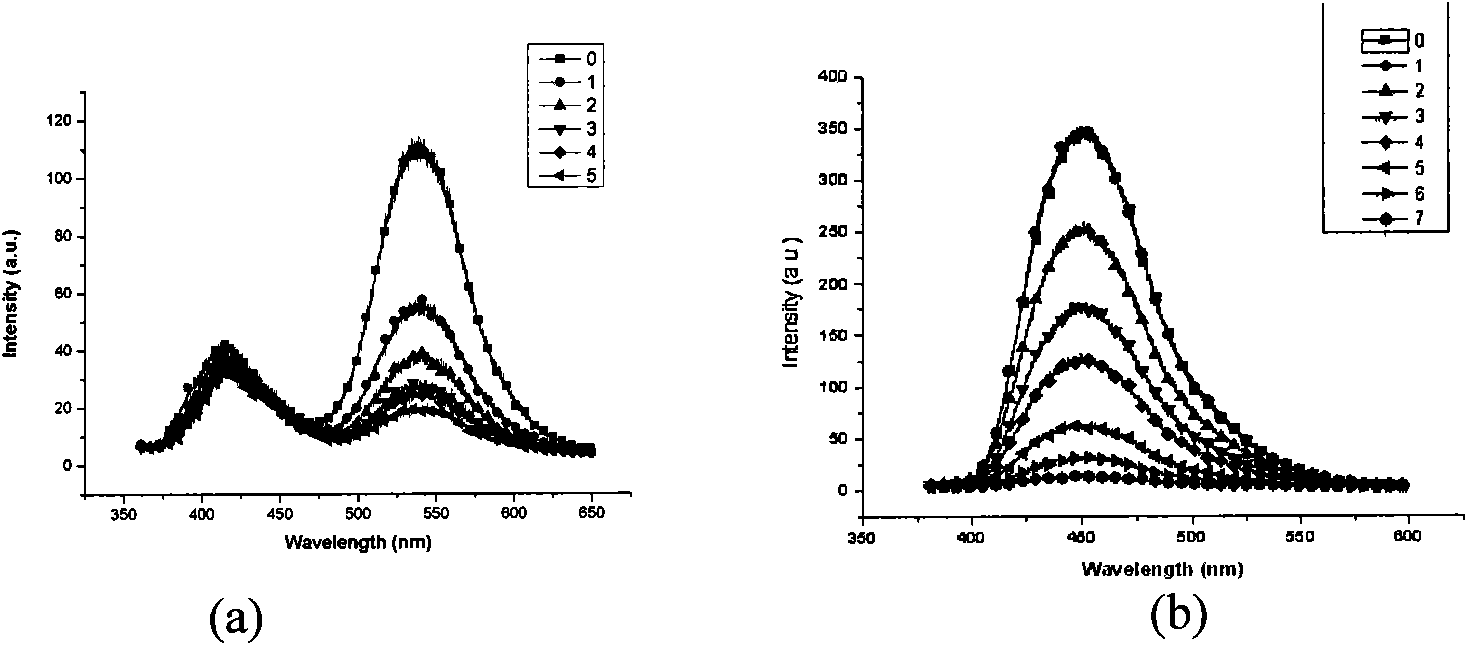
Fluorescent sensing material with sensing function to phenyl amine compounds, method and application thereof
A fluorescence sensing and compound technology, applied in the field of fluorescence sensing materials, can solve the problems of slow detection, low sensitivity, and complicated pretreatment of detection methods, and achieve the effects of easy film formation, good selectivity, and easy mass synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 (synthesis of TPA-Py)
[0046]
[0047] Under argon protection, 1.66g (4.47mmol) triphenylamine boric acid, 0.26g Pd(PPh 3 ) 4 (0.22mmol), 2.05g (14.86mmol) K 2 CO 3 , 7.44 ml H 2 O, 0.354 ml (3.71 mmol) of 2-bromopyridine and 30 ml of tetrahydrofuran were heated to 85° C. to react overnight. Extract with dichloromethane, and combine the organic phases. Washed with water, washed with saturated brine, anhydrous MgSO 4 dry. The yellow powder separated by column chromatography was 1.23 g, and the yield was 90%. 1 H NMR (500MHz CDCl 3 ): δ8.66(d, 1H), 7.87(d, 2H), 7.72(t, 1H), 7.67(d, 1H), 7.26(t, 3H), 7.18(t, 1H), 7.14(6H) , 7.04(t,2H), .MALDI-TOF MS m / z: 322
Embodiment 2
[0048] Embodiment 2 (synthesis of Br-BTD-Py)
[0049]
[0050] Under argon protection, 5.48g (18.64mmol) of 4,7-dibromo-2,1,3-benzothiadiazole, 1.07g Pd(PPh 3 ) 4 (0.93mmol), 6.86g 2-tributyltinylpyridine (18.64mmol), dissolved in 100ml of toluene. Heated to 125°C for 3 days. Extract with dichloromethane, and combine the organic phases. Washed with water, washed with saturated brine, anhydrous MgSO 4 Dry and separate by column chromatography to obtain 1.5 g of yellow powder with a yield of 27.6%. 1 H NMR (500MHz CDCl 3 ): δ8.77(d, 1H), 8.63(d, 1H), 8.36(d, 1H), 8.00(d, 1H), 7.87(t, 1H), 7.34(t, 1H). MALDI-TOF MS m / z: 292
Embodiment 3
[0051] Embodiment 3 (synthesis of TPA-BTD-Py)
[0052]
[0053] Under argon protection, 0.763g (1.17mmol) triphenylamine boric acid, 0.18g Pd(PPh 3 ) 4 (0.16mmol), 0.88g (6.37mmol) K 2 CO 3 , 3.16mL H 2 O, 0.463g (1.58mmol) of 4-bromo-7-pyridyl-2,1,3-benzothiadiazole was dissolved by adding 35mL of toluene. To was heated to 125° C. to react overnight, the reaction was stopped, extracted with dichloromethane, and the organic phases were combined. Washed with water, washed with saturated brine, anhydrous MgSO 4 dry. Column chromatography separated 0.55 g of red powder with a yield of 76%. 1 H NMR (500MHz CDCl 3 ): δ8.79(d, 1H), 8.69(d, 1H), 8.52(d, 1H), 7.88(3H), 7.83(d, 1H), 7.33(d, 1H), 7.28(t, 4H) , 7.19(t, 6H), 7.06(t, 2H). MALDI-TOF MS m / z: 456
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com