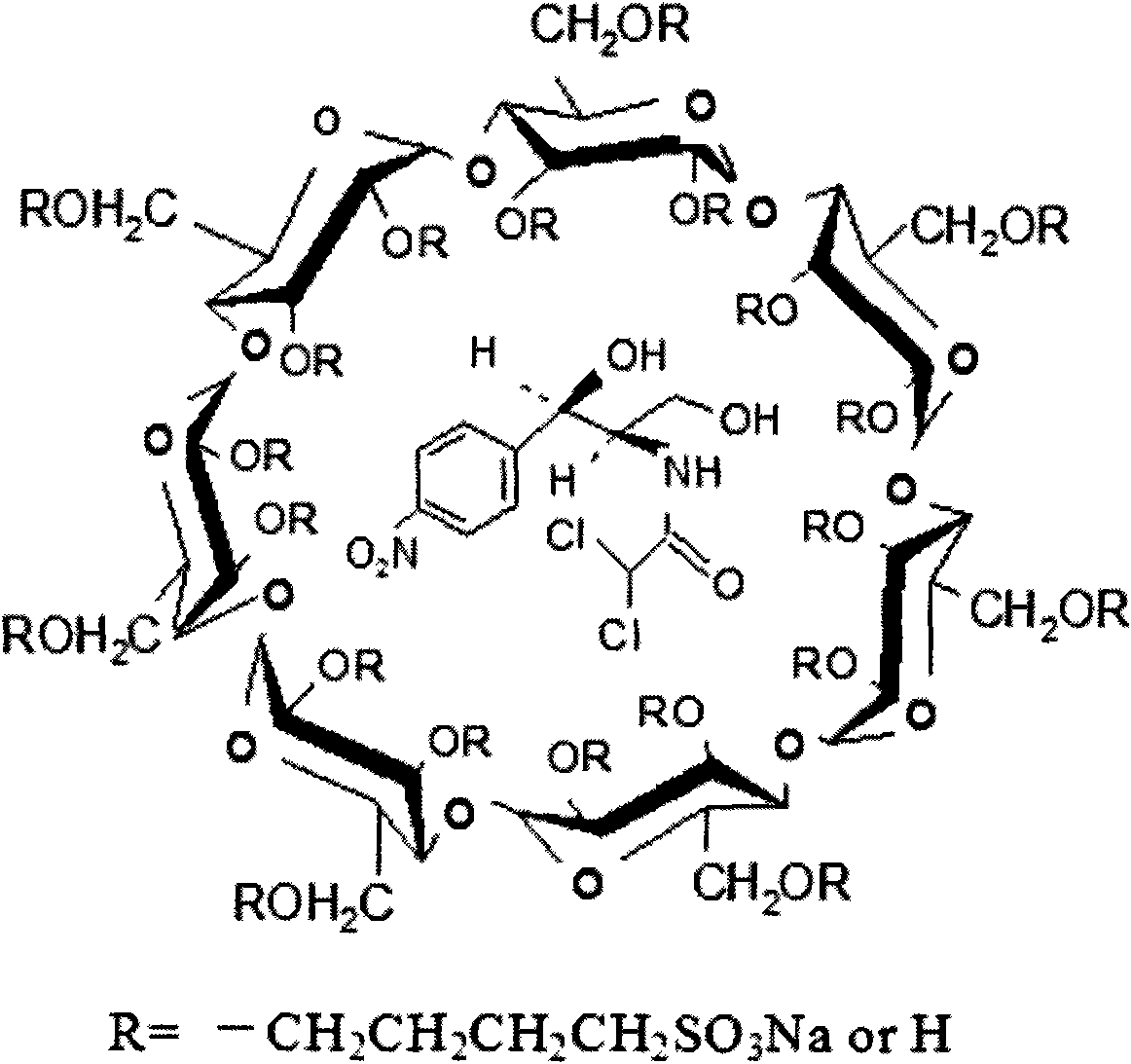
Application of the sulfur-butyl ether-belta-cyclodextrin in eyedrops of chloramphenicol and method thereof
A technology of sulfobutyl ether and application method, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve the problems of non-conformity with quality requirements, low solubility, and chloramphenicol eye drops. Solve problems such as agent precipitation, achieve thermal stability, improve stability, and reduce hemolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Drug composition:
[0108] Chloramphenicol 2.625g / L
[0109] Borax 0.625g / L
[0110] Boric acid 17g / L
[0111] Phenylmercury acetate 0.02g / L
[0112] SBE-β-CD 20g / L
[0113] Water up to 1L
[0114] Standard: the content of chloramphenicol diol shall not exceed 8%, and p-nitrobenzaldehyde shall not exceed 0.5%.
[0115] Preparation method:
[0116] 1. Put the formula amount of chloramphenicol and SBE-β-CD into 150mL water, stir for 30 minutes to fully dissolve and mix evenly, then place it for later use;
[0117] 2. Put the formula amount of borax and boric acid into 300mL water, stir to dissolve them fully, and set aside;
[0118] 3. Put the formula amount of phenylmercuric acetate (or benzalkonium bromide, thimerosal) into 500mL water, stir well to dissolve it, place it for later use;
[0119] 4. Put the solutions in the above steps 1, 2, and 3 together into a container, stir for 10 minutes to mix them evenly, add water to 1L, fill them into a small bottle, and...
Embodiment 2
[0121] In the drug component, the dosage of SBE-β-CD is 10g / L, and the other ingredients and preparation method are the same as in Example 1.
Embodiment 3
[0123] In the drug component, the dosage of SBE-β-CD is 5g / L, and the other ingredients and preparation method are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com