Variable region sequence for novel anti-beta amyloid polypeptide antibody and coding sequence thereof
A kind of variable region, antibody technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Screening of monoclonal phage antibodies:
[0025] Multiple rounds of enrichment and screening of human phage single-chain antibody libraries were performed using phage display technology, and four rounds of "adsorption-elution-amplification" enrichment screening were performed using purified Alzheimer's disease Aβ as the antigen. The recovery rate of rounds of screening showed an increasing trend, indicating that after screening, the phages with Aβ specificity were highly enriched (Table 1). Methods as below:
[0026] (1) Amplification of the phage antibody library: Take 100 μl of the original library bacteria solution, inoculate it into 100ml 2×YT-AG (2×YT containing 100 μg / ml ampicillin, 1% glucose) medium, and culture it with shaking at 37°C until Logarithmic period. plus 10 12 The pfu (plaque forming unit) helper phage M13KO7 was placed in the culture medium, bathed in water at 37°C for 30min, centrifuged at 4°C and discarded the supernatant. Resuspe...
Embodiment 2
[0032] Example 2: Detection of monoclonal phage antibodies:
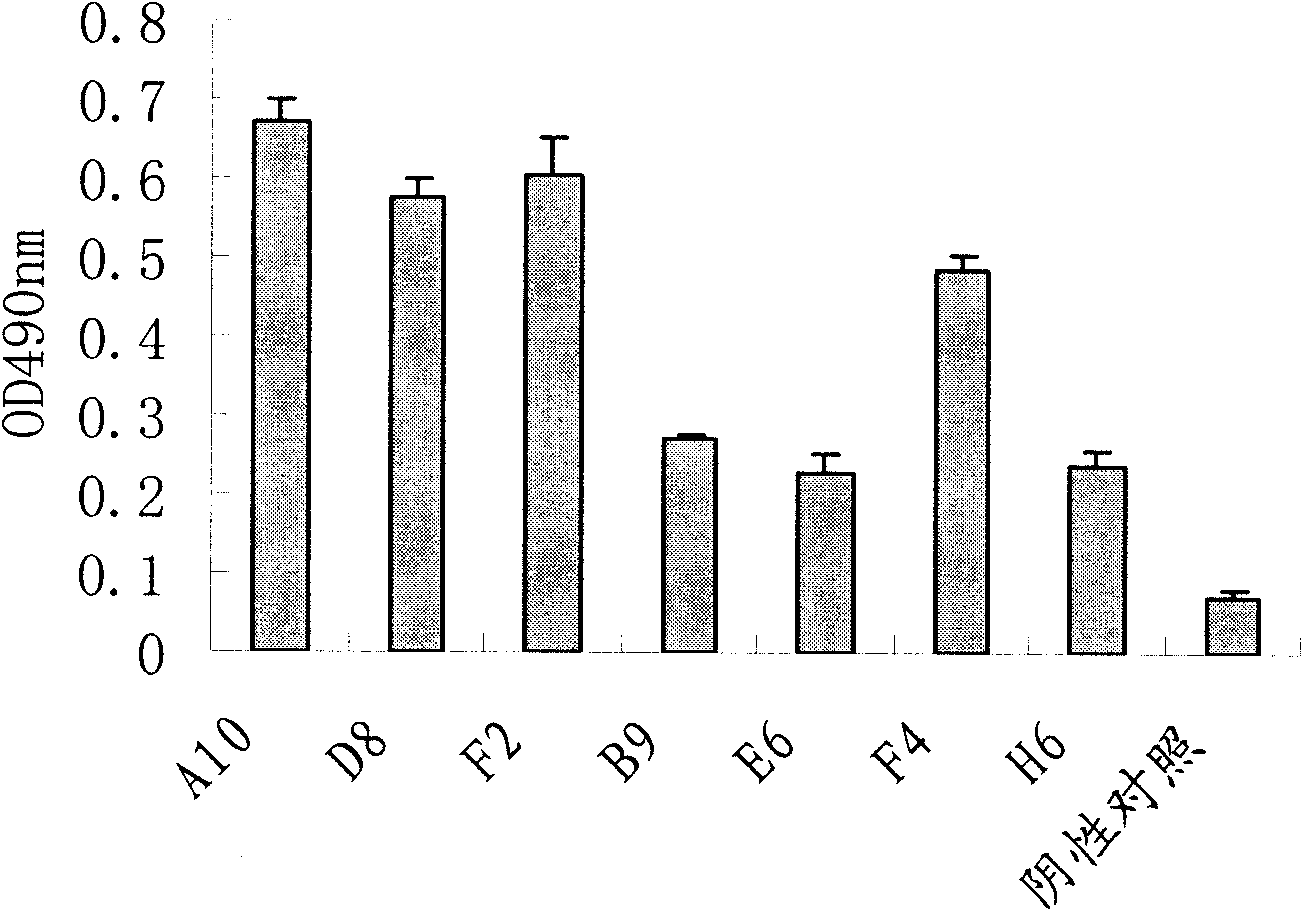
[0033] 80 single bacterial clones were randomly picked from the TYE-AG plate cultured overnight after the fourth round of screening, and cultured to prepare phage antibodies. ELISA method for detection: Antigen Aβ was coated on an enzyme plate (0.4 μg / well), and after blocking, 100 μl of monoclonal phage antibody supernatant was added to each well, and incubated at 37° C. for 2 hours. After washing, 1:5000 HRP-labeled mouse anti-M13 antibody was added and incubated at 37°C for 1h. The positive control was the primary antibody (mouse anti-human Aβ), the secondary antibody (goat anti-mouse IgG); the negative control only added 2% MPBS. With OPD as substrate, 2M sulfuric acid was used to terminate the reaction, and detected by OD490nm of microplate reader. When the OD value was greater than 2 times of the negative control, it was judged as positive. Detection results of monoclonal phage antibody binding to Aβ antige...
Embodiment 3
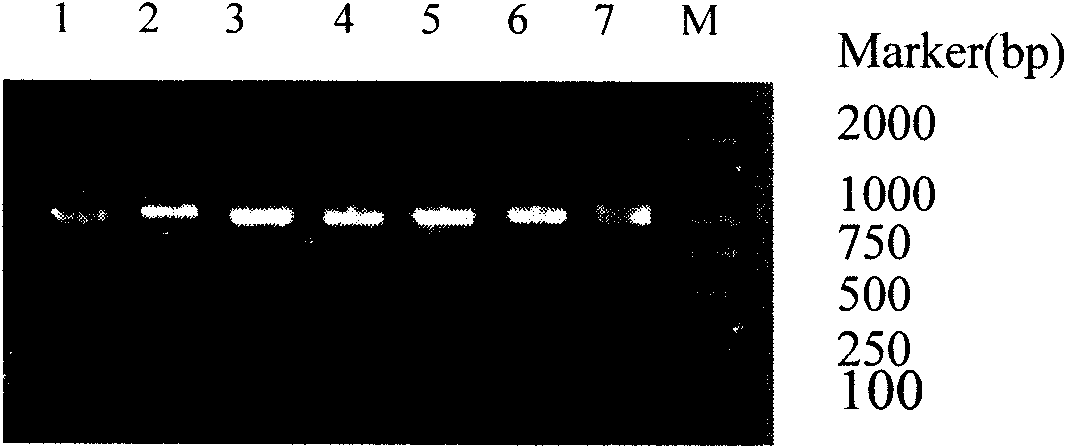
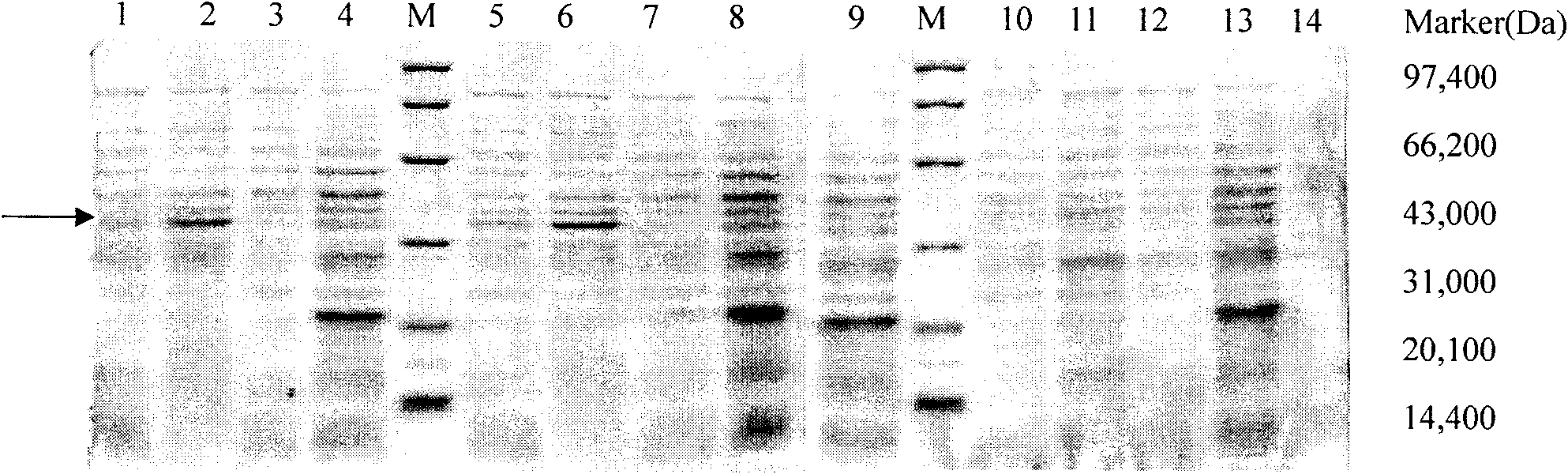
[0035] Example 3: Expression and identification of soluble anti-Aβ scFv fragments:
[0036] (1) The expression of positive phagemids was induced in Escherichia coli HB2151, and the phages displaying antibodies specifically binding to Aβ were infected with logarithmic phase Escherichia coli HB2151, and left in a water bath at 37°C for 30 minutes. Inoculate on SOBAG-N plates and culture at 30°C until a single colony grows. Pick one clone from each plate with different clone sources, inoculate them in 5ml 2×YT-AG medium respectively, and shake overnight at 30°C. Add 5ml of overnight bacteria to 50ml of fresh 2×YT-AG medium, shake at 30°C for 1h. Centrifuge, discard the supernatant, resuspend the pellet in 50ml of fresh 2×YT-AI (I:IPTG, 1mM) medium, induce expression at 30°C for 20h. After centrifugation, the supernatant was sterilized by filtration and stored at -20°C. Resuspend one tube of pellet with 0.5ml ice-cold 1×TES, then add 0.75ml ice-cold 1 / 5×TES, vortex, and ice-bat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com