Application of compound as JAK-STAT3 signal passage inhibitor
A technology of JAK-STAT3 and signaling pathways, which is applied in the direction of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems that have not yet been clinically treated for cancer, and achieve the effect of improving prognosis and improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
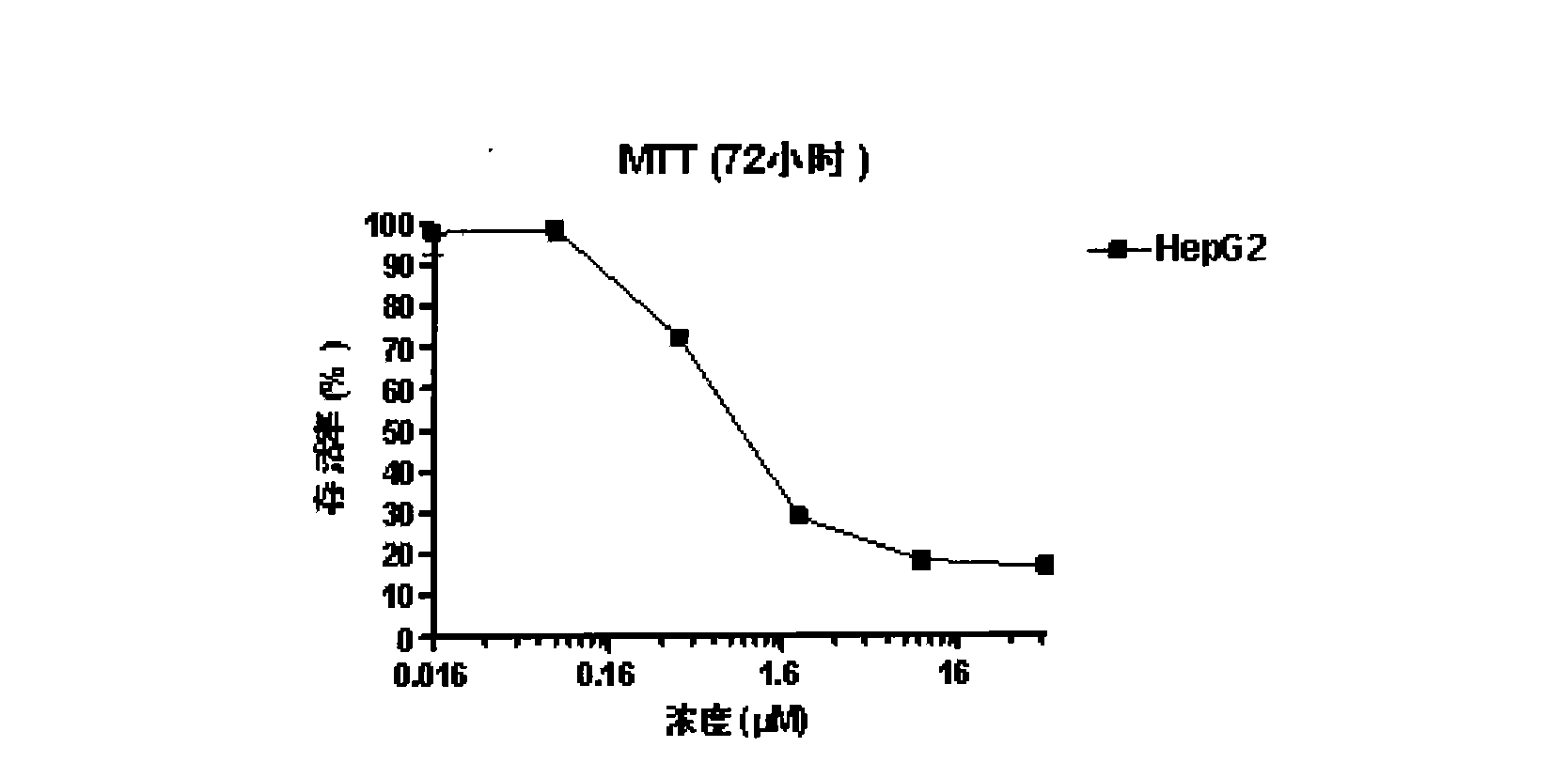
Image
Examples
Embodiment 1
[0150] 5-Chloro-N-(4-fluorophenyl)-2-hydroxybenzamide
[0151] (5-chloro-N-(4-fluorophenyl)-2-hydroxybenzamide)
[0152]
[0153] resolve resolution:
[0154] Mix 1 mmol of 5-chloro-2-methoxybenzoic acid and 2 ml of thionyl chloride and heat to reflux for one hour. Thionyl chloride was distilled off, then diluted with 2 ml of anhydrous tetrahydrofuran, and then used.
[0155] 2 mmol of 4-fluoroaniline compounds were dissolved in 2 ml of anhydrous tetrahydrofuran, and then added dropwise to the previously prepared 5-chloro-2-methoxybenzoyl chloride solution at room temperature, and stirred overnight at room temperature. After the reaction was detected by thin layer chromatography, at room temperature, an appropriate amount of water and dichloromethane were added, the mixture was transferred to a separatory funnel, and the organic layer was taken. The organic layer solution was washed successively with appropriate amount of dilute sodium hydroxide solution (once), saturate...
Embodiment 2
[0160] 5-Chloro-N-(3,4-difluorophenyl)-2-hydroxybenzamide
[0161] (5-chloro-N-(3,4-difluorophenyl)-2-hydroxybenzamide)
[0162]
[0163] The synthesis method is as in Example 1.
[0164] 1 HNMR (400MHz, DMSO-d 6 ), δ11.60(s, 1H), 10.52(s, 1H), 7.86~7.91(m, 2H), 7.43~7.48(m, 3H), 7.46~7.49(m, 1H), 7.02(d, J = 1.6Hz, 1H)
[0165] MS(ES+APCI), m / z: 284.1(M+H) + , 282.0(M-H) -
Embodiment 3
[0167] 5-Chloro-N-(4-(4-morpholinyl)phenyl)-2-hydroxybenzamide
[0168]
[0169] The synthesis method is as in Example 1.
[0170] 1 HNMR (400MHz, DMSO-d 6 ), δ10.41(s, 1H), 7.99(d, J=2.0Hz, 1H), 7.54(d, J=8.8Hz, 2H), 7.43~7.46(m, 1H), 6.94~6.99(m, 3H),
[0171] MS(ES+APCI), m / z: 333.0(M+H) + , 331.2(M-H) -
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com