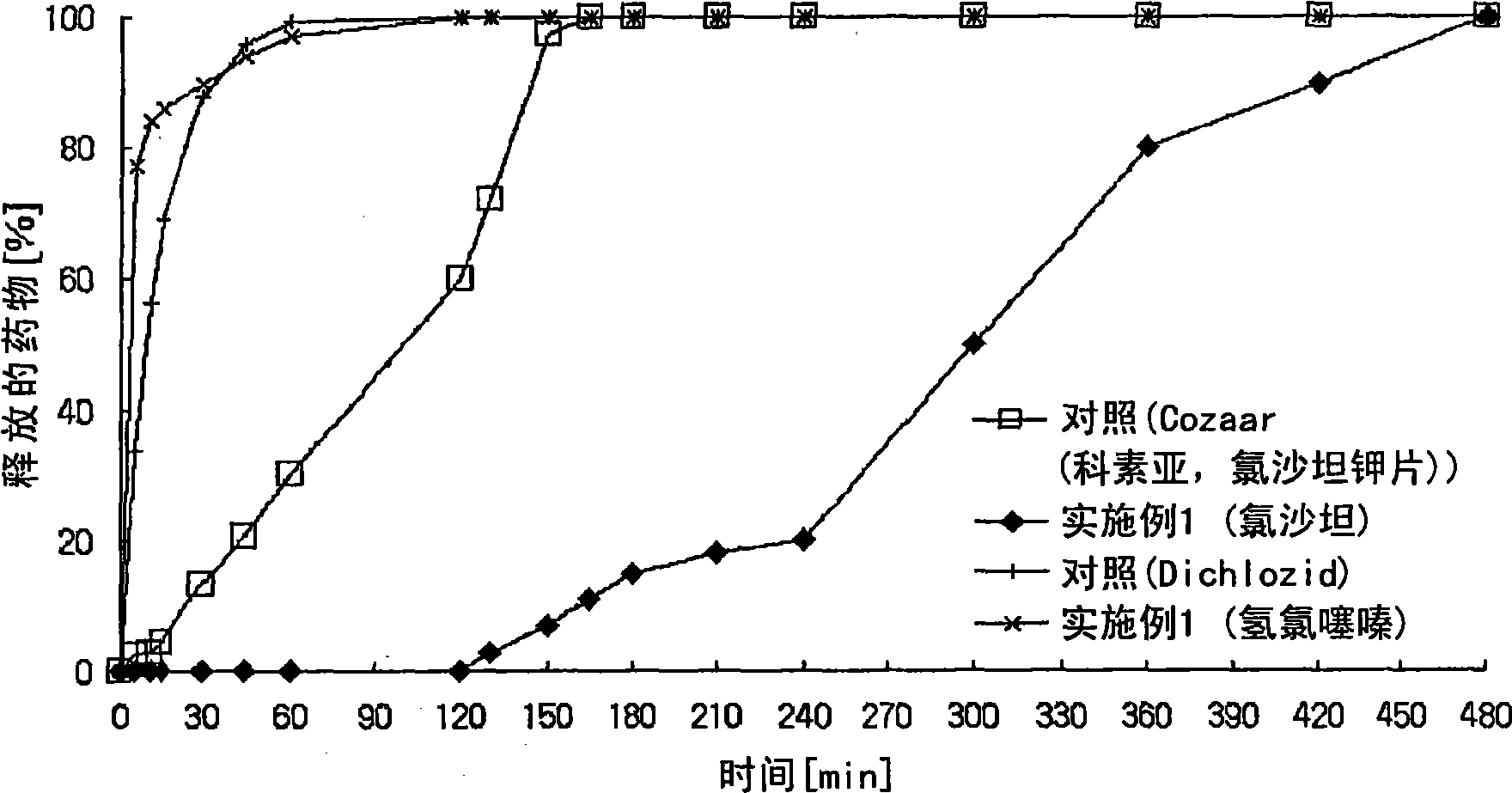
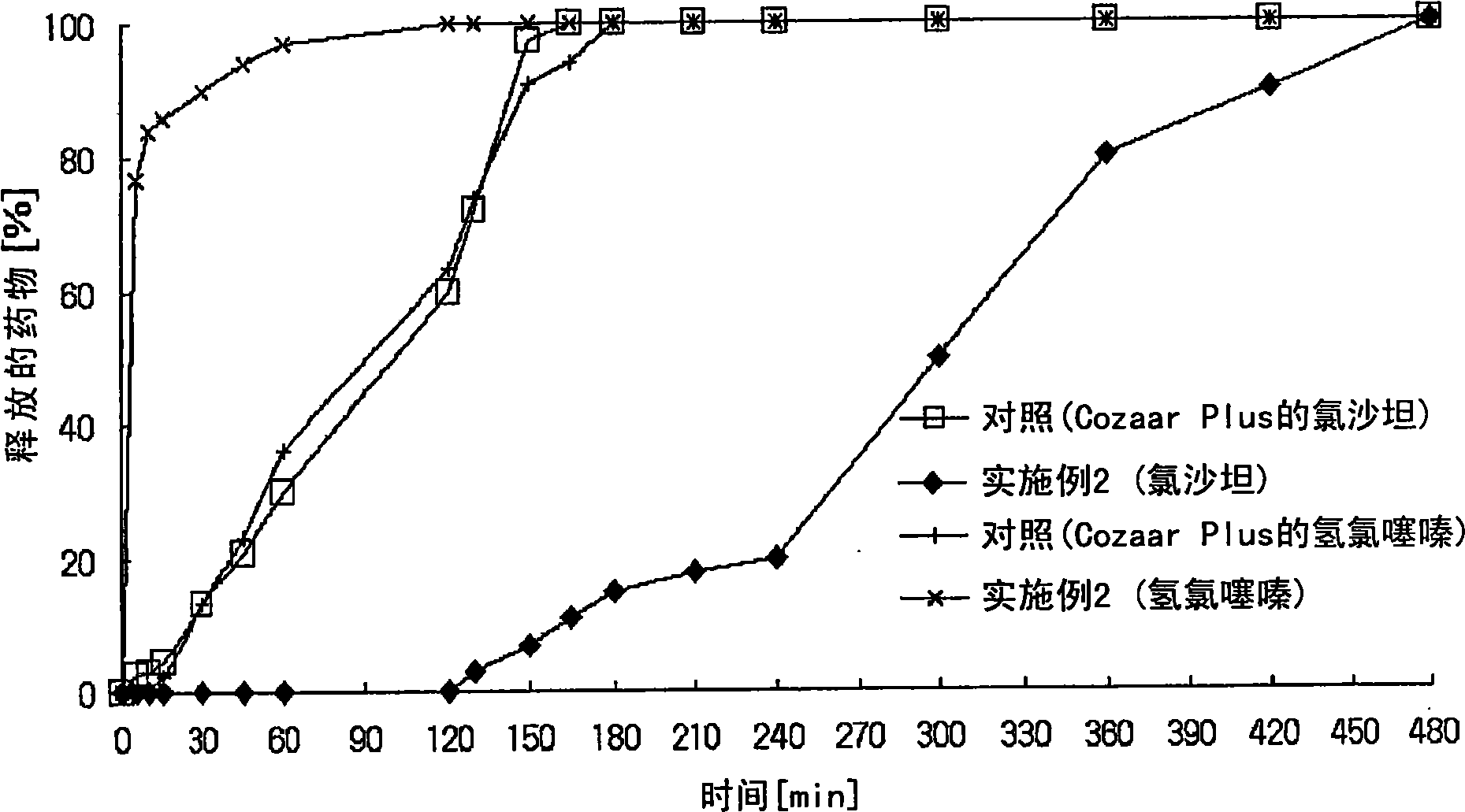
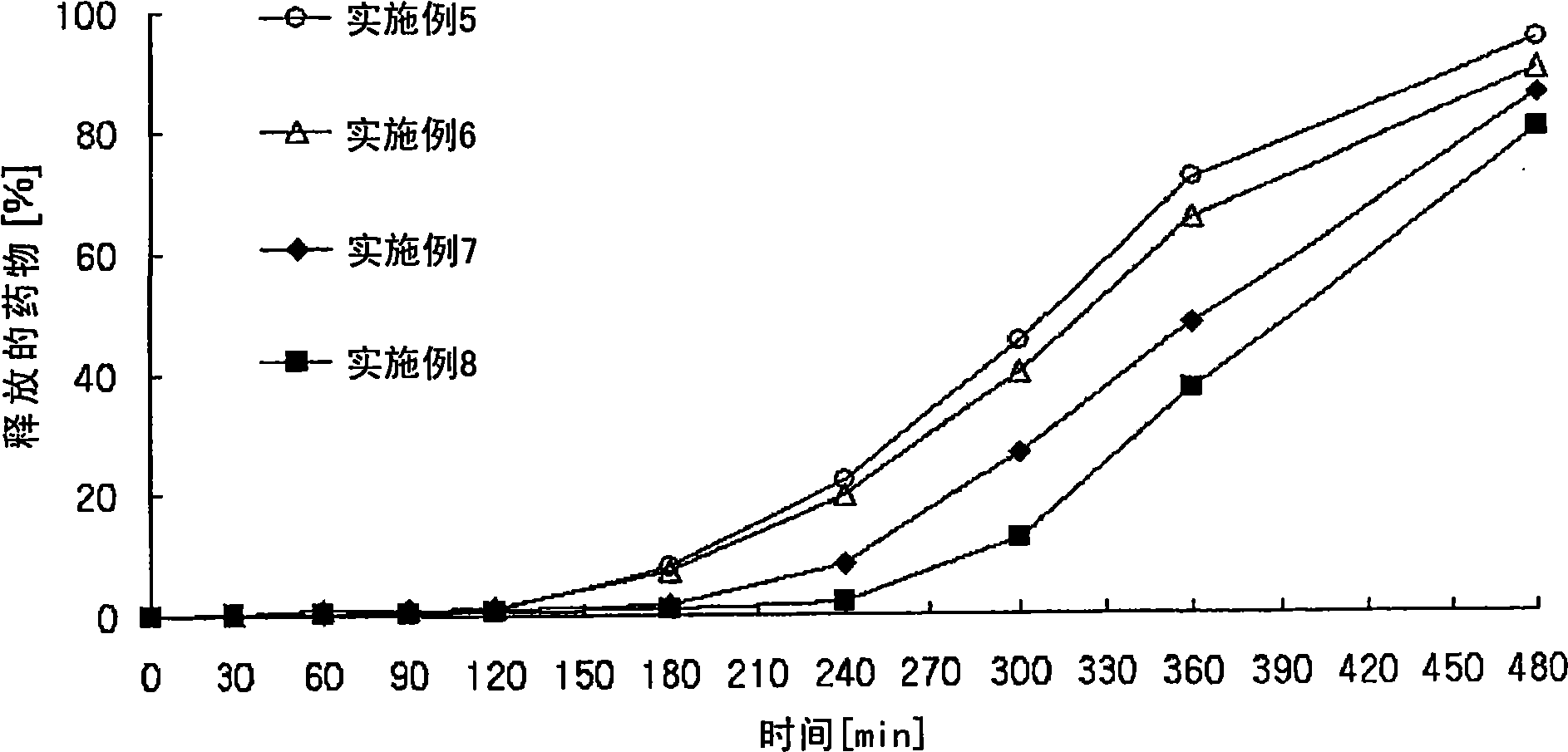
Controlled release pharmaceutical composition containing thiazides and angiotensin-ii-receptor blockers
A technology of angiotensin and receptor blockers, applied in the field of blockers or pharmaceutically acceptable drug combination preparations of thiazide compounds and angiotensin II receptor blockers, which can solve the problem of insufficiently shown Antihypertensive and preventive effects of complications, lack of creativity, increased side effects and other issues, to achieve the effect of maintaining antihypertensive effects, improving patient compliance, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 14
[0145] Examples 1 to 14: Preparation of dry-coated tablets
[0146] 1) Preparation of losartan delayed-release tablet core
[0147] To prepare the losartan delayed-release tablet core, as shown in Table 4, losartan potassium, microcrystalline cellulose, pregelatinized starch, copovidone and micropowder silica gel 2000 (Aerosil2000) were sieved through a No. 35 sieve and mixed with each other in a high-speed mixer for 5 minutes to prepare a mixture. The magnesium stearate was mixed with the mixture for 4 minutes. The resulting mixture was compressed into core tablets using a rotary tablet press (MRC-33, Sejong Machinery Co., Korea). The thus-prepared core tablets were placed in a Hi-coater (SFC-30N, Sejong Machinery Co., Korea) to prepare delayed-release core tablet products having the composition and content shown in Table 4.
[0148] 2) Preparation of hydrochlorothiazide immediate-release layer
[0149] To prepare the hydrochlorothiazide immediate-release layer, hydrochlo...
Embodiment 15 to 22
[0152] Examples 15 to 22: Preparation of multilayer tablets
[0153] 1) Preparation of losartan delayed-release layer
[0154] In Example 15, in order to prepare losartan delayed-release tablet core, losartan potassium, microcrystalline cellulose, pregelatinized starch, copovidone and sodium starch glycolate were sieved through a No. 35 sieve and Mix with each other in a high speed mixer for 5 minutes to prepare a mixture. Meanwhile, hydroxypropyl cellulose and hydroxypropyl cellulose phthalate (HP-50) were dissolved in pure water to prepare a binder solution. A binder solution is added to the mixture, which is then kneaded, granulated and dried. The dried granules were placed in a fluid bed coater. Meanwhile, hydroxypropylcellulose phthalate (HP-55) and polyethylene glycol 6000 were dissolved in 220 mg of ethanol and 980 mg of methylene chloride to prepare a coating solution. The granules were coated with a coating solution in a fluidized bed coater (GPCG-1, Glatt, German...
Embodiment 23
[0160] Example 23: Preparation of film-coated tablets
[0161] 1) Preparation of losartan delayed-release layer
[0162] As shown in Table 5 below, losartan potassium, microcrystalline cellulose, sodium starch glycolate, and lactose were sieved through a No. 35 sieve and mixed with each other in a high-speed mixer for 5 minutes to prepare a mixture. Meanwhile, hydroxypropyl cellulose and hydroxypropyl cellulose phthalate (HP-50) were dissolved in pure water to prepare a binder solution. A binder solution is added to the mixture, which is then kneaded, granulated and dried. Place the dried granules in a fluid bed coater. Meanwhile, hydroxypropylcellulose phthalate (HP-55) and polyethylene glycol 6000 were dissolved in 220 mg of ethanol and 980 mg of methylene chloride to prepare a coating solution. The granules were coated with a coating solution in a fluidized bed coater (GPCG-1, Glatt, Germany). After the coating process was completed, micropowder silica gel 200 was mixed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com