Green electroluminescent compounds and organic electroluminescent device using the same
A compound and electroluminescence technology, applied in electroluminescence light sources, preparation of organic compounds, silicon organic compounds, etc., can solve the problem of not mentioning the 2- and 7-position substituted asymmetric compounds of diarylamino groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
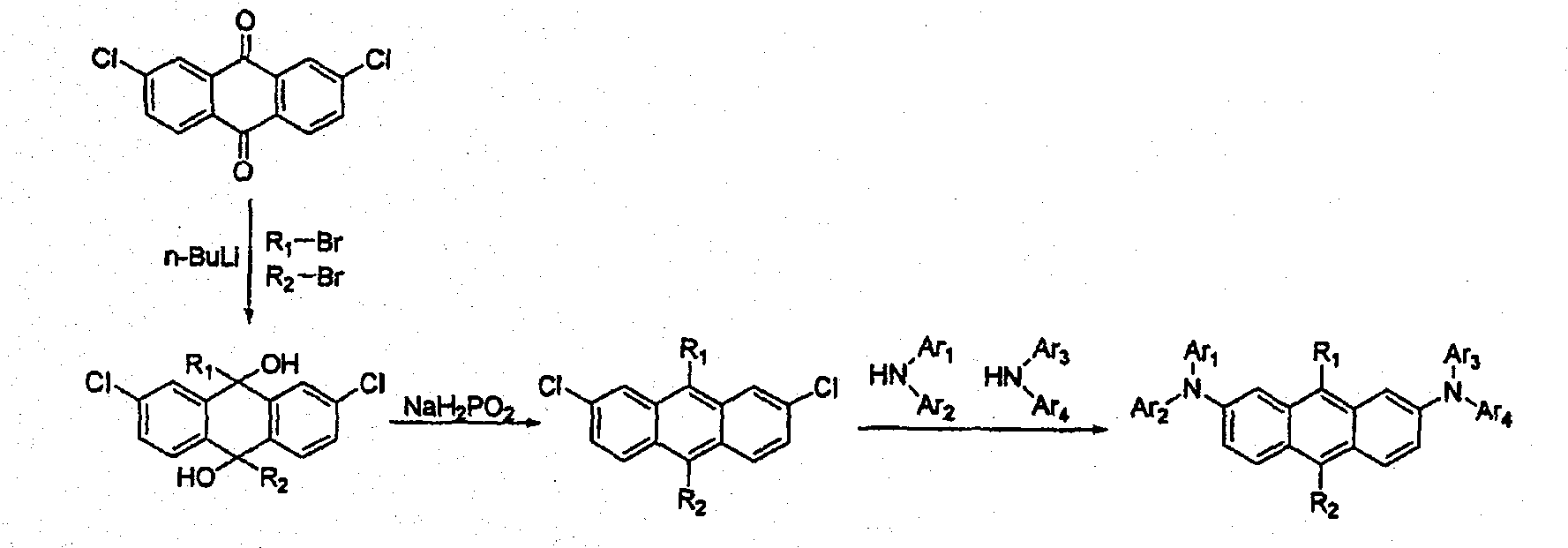
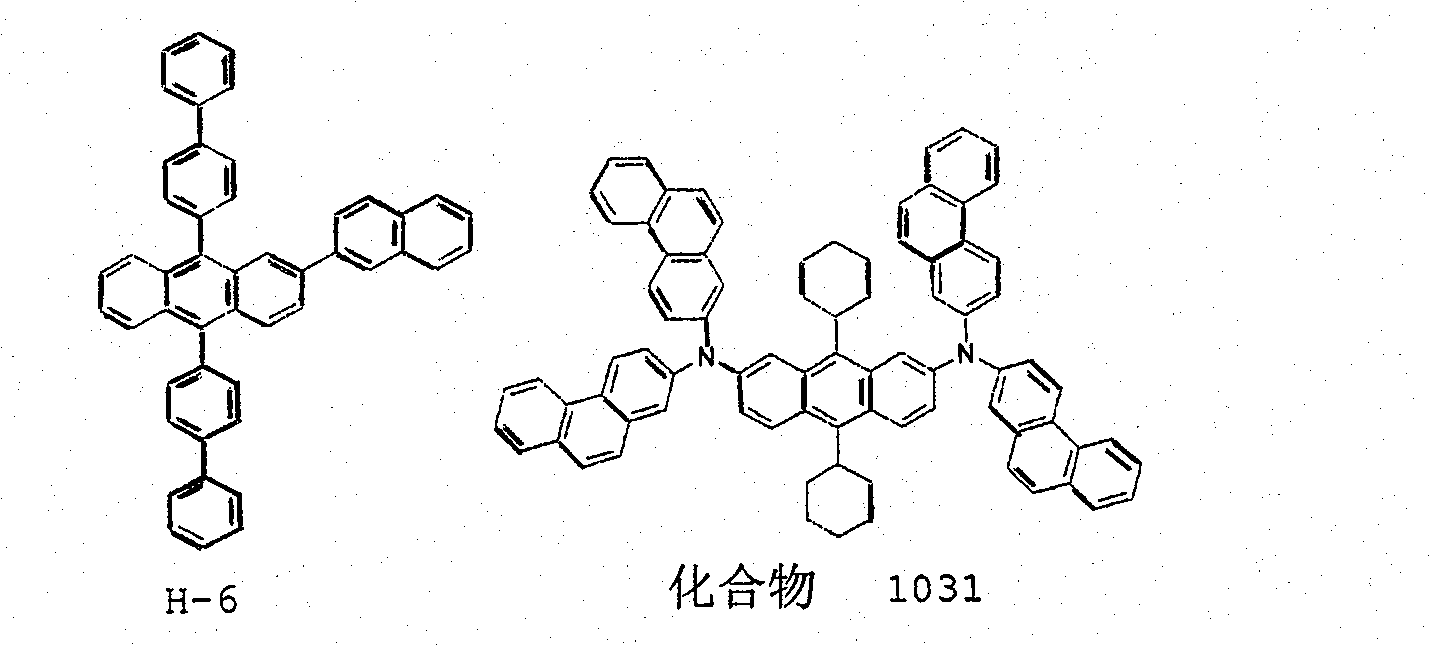
[0210] [Preparation Example 1] Preparation of Compound (1023)
[0211]
[0212] Preparation of compound (A)
[0213] Bromocyclohexane (44.1g, 270.7mmol) was dissolved in dry tetrahydrofuran solvent (500ml), and 2.5M n-butyllithium (in n-hexane) (130mL, 324.9mmol) was added at -78°C in. After stirring for 1 hour, 2,7-dichloroanthracene-9,10-dione (30.0 g, 108.3 mmol) was added thereto, and the resulting mixture was stirred while slowly warming to room temperature. After 17 hours, water was added, the mixture was stirred for 30 minutes and extracted with 500 mL of vinyl acetate. The extract was washed with 500 mL of water, and the organic layer was dried over magnesium sulfate, and concentrated under reduced pressure. The residue was dried to obtain compound (A) (21.3 g, 47.8 mmol).
[0214] Preparation of compound (B)
[0215] Compound (A) (21.3g, 47.8mmol), potassium iodide (31.7g, 191.2mmol), sodium hydrogen phosphate (40.5g, 382.4mmol) and acetic acid (300mL) wer...
Embodiment 1
[0591] [Example 1] Preparation of OLED using the organic EL compound of the present invention
[0592] OLED devices were prepared using the EL materials of the present invention.
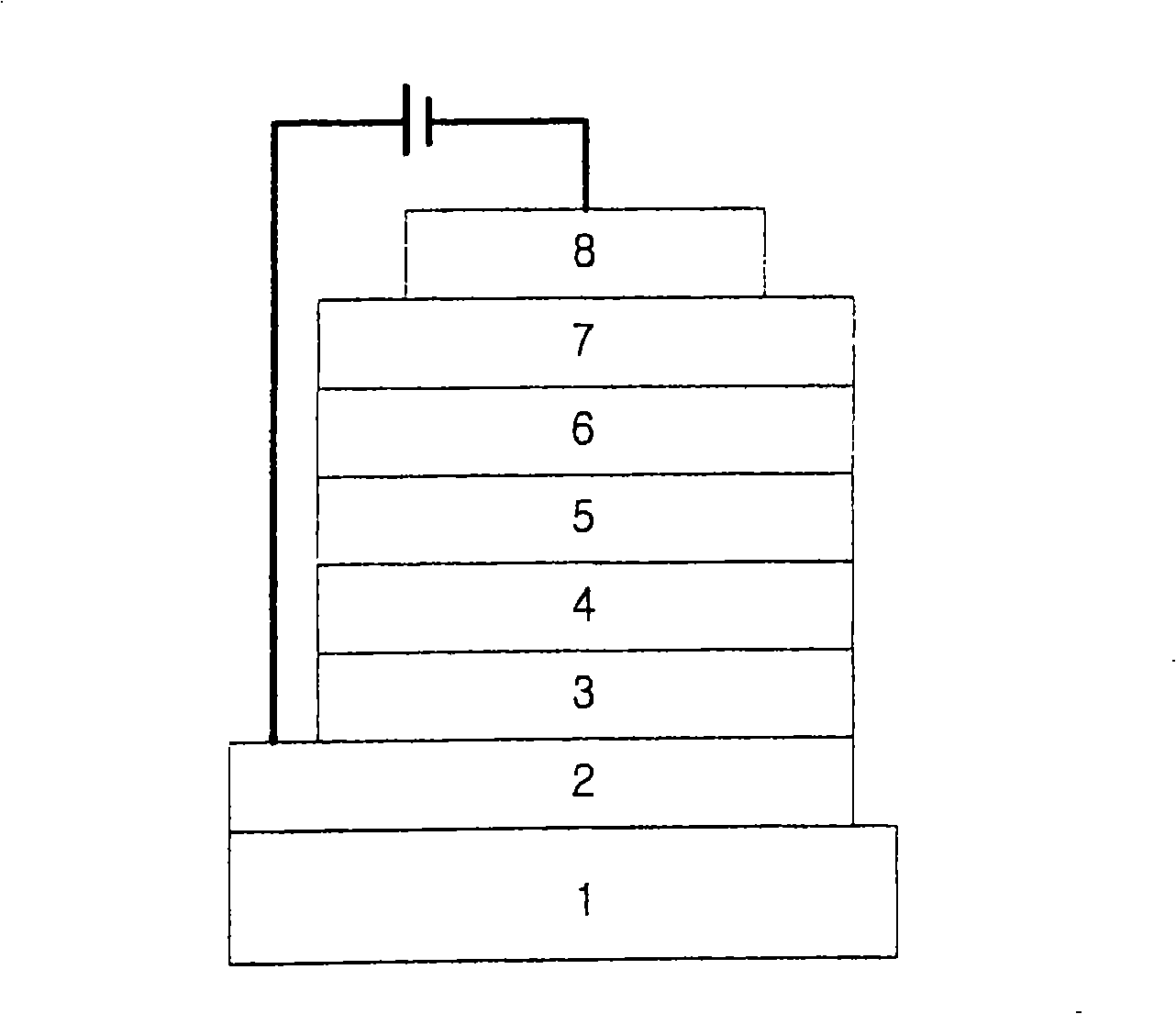
[0593] First, a transparent electrode ITO film (15Ω / □) (2) prepared from glass for OLED (manufactured by Samsung Corning) (1) was ultrasonically washed in trichloroethylene, acetone, ethanol, and distilled water, Next, store it in isopropanol until use.
[0594] Then, the ITO substrate was mounted on the substrate holder of the vacuum vapor deposition device, and 4,4',4"-tris(N,N-(2-naphthyl)-phenylamino)triphenylamine (2 -TNATA) is placed in a unit of the vacuum vapor deposition device, and then the exhaust gas makes the vacuum degree in the reaction chamber up to 10 -6 torr. A current was applied to the cell to evaporate 2-TNATA, providing vapor deposition of a 60 nm thick hole injection layer (3) on the ITO substrate.
[0595]
[0596] Then, inject N,N'-bis(α-naphthyl)-N,N'-diphenyl-4,4'-d...
Embodiment 2
[0612] [Example 2] The EL performance of the prepared OLED
[0613] respectively at 5000cd / m 2 and 20000cd / m 2 The luminous efficiencies of the OLEDs prepared in Example 1 and Comparative Examples 1-2 were measured as follows. The OLEDs prepared in Example 1 and Comparative Examples 1-2 included the organic EL compound of the present invention and the traditional electroluminescent compound respectively. The results are shown in Table 3. Since the electroluminescent performance in the high brightness region is very important, especially for green electroluminescent materials, the high brightness (about 20000cd / m 2 )The data.
[0614] table 3
[0615]
[0616] As can be seen from Table 3, when the organic EL compound of the present invention is used in a green electroluminescent device, the device exhibits a significantly improved Luminous efficiency.
[0617] Specifically, even at high brightness (20000cd / m 2 ), the efficiency reduction of the high-performance electro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com