Rapid diagnosis reagent kit for detecting four/more than four invasive candidiasis
A technology for rapid diagnosis and application of methods, applied in the direction of microorganism-based methods, biochemical equipment and methods, fluorescence/phosphorescence, etc., can solve the problems of inappropriate tissue biopsy methods, low positive rate, unacceptable patients, etc., to reduce deaths rate and sequelae, high detection sensitivity, and highly reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0031] This embodiment 1 constitutes a fluorescent quantitative PCR rapid diagnostic kit for detecting four kinds of invasive candida and its application method. It includes a box body and a fluorescent quantitative PCR reaction solution arranged in the box body, positive quality control products and negative quality control products of four kinds of Candida; the fluorescent quantitative PCR reaction solution contains specific primers and fluorescent lights corresponding to four kinds of Candida Probe; The positive quality control product of described invasive Candida is the DNA sample obtained by "boiling method" from the standard bacterial strain of Candida albicans, Candida krusei, Candida glabrata, and Candida tropicalis, and the negative quality control product for sterilized triple distilled water.
[0032] The preparation method of described three kinds of substances is as follows:
[0033] 1) Preparation of fluorescent quantitative PCR reaction solution: 12.5 μl of Br...
specific Embodiment 2
[0050] Example 2 constitutes a fluorescent quantitative PCR rapid diagnostic kit for detecting four / more than four types of invasive Candida and its application method.
[0051] The characteristics of this specific embodiment 2 are: the positive quality control product of described invasive Candida is made of Candida albicans, Candida krusei, Candida glabrata, Candida tropicalis plus several other standard bacterial strains of invasive Candida The DNA sample obtained by "boiling method", the negative quality control substance of the invasive Candida is sterilized triple distilled water. The other invasive Candida can be one or a combination of two or more of Candida parapsilosis, Candida quaternidae, Candida portuguese and Candida lactis. All the other are identical with specific embodiment 1.
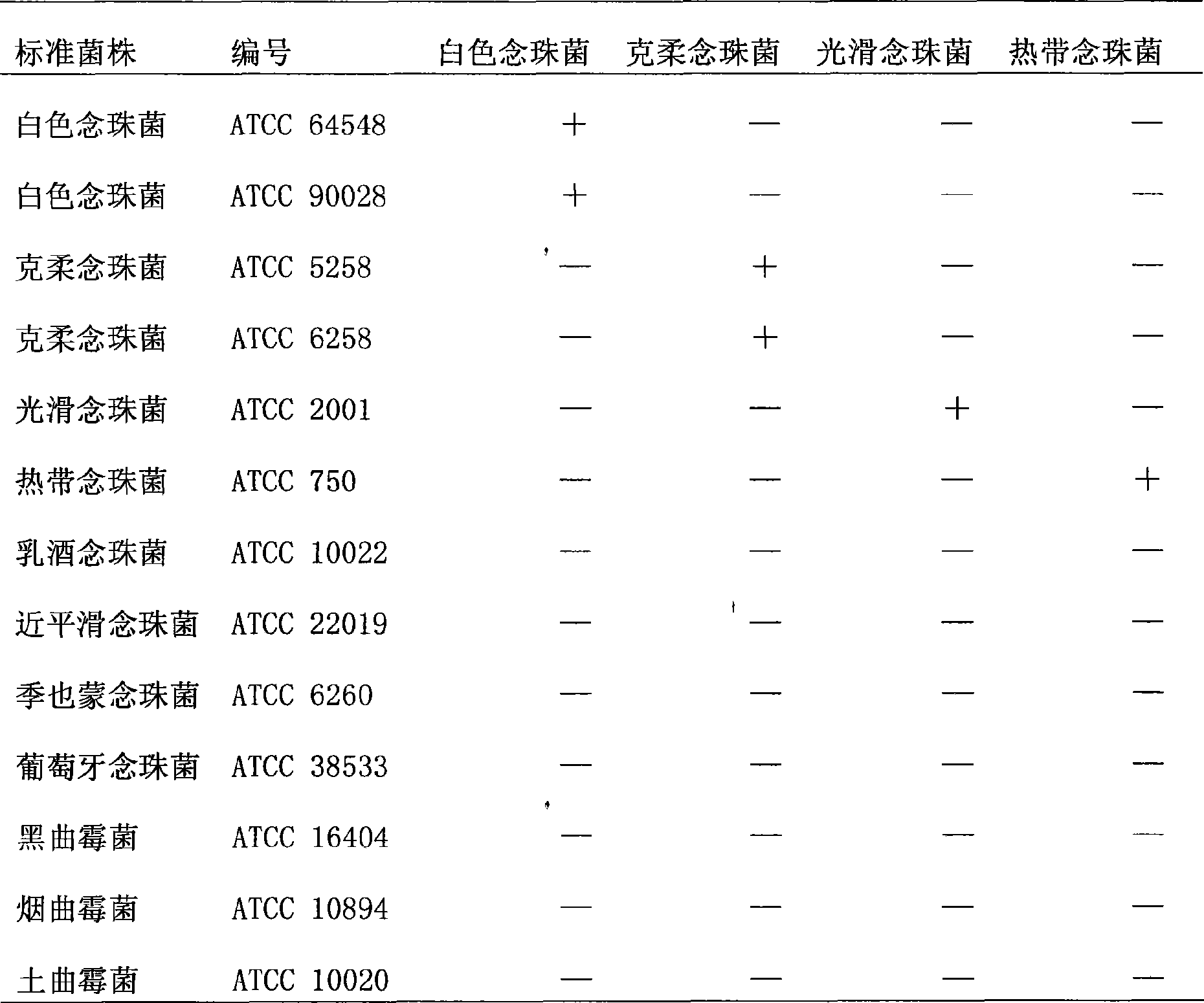
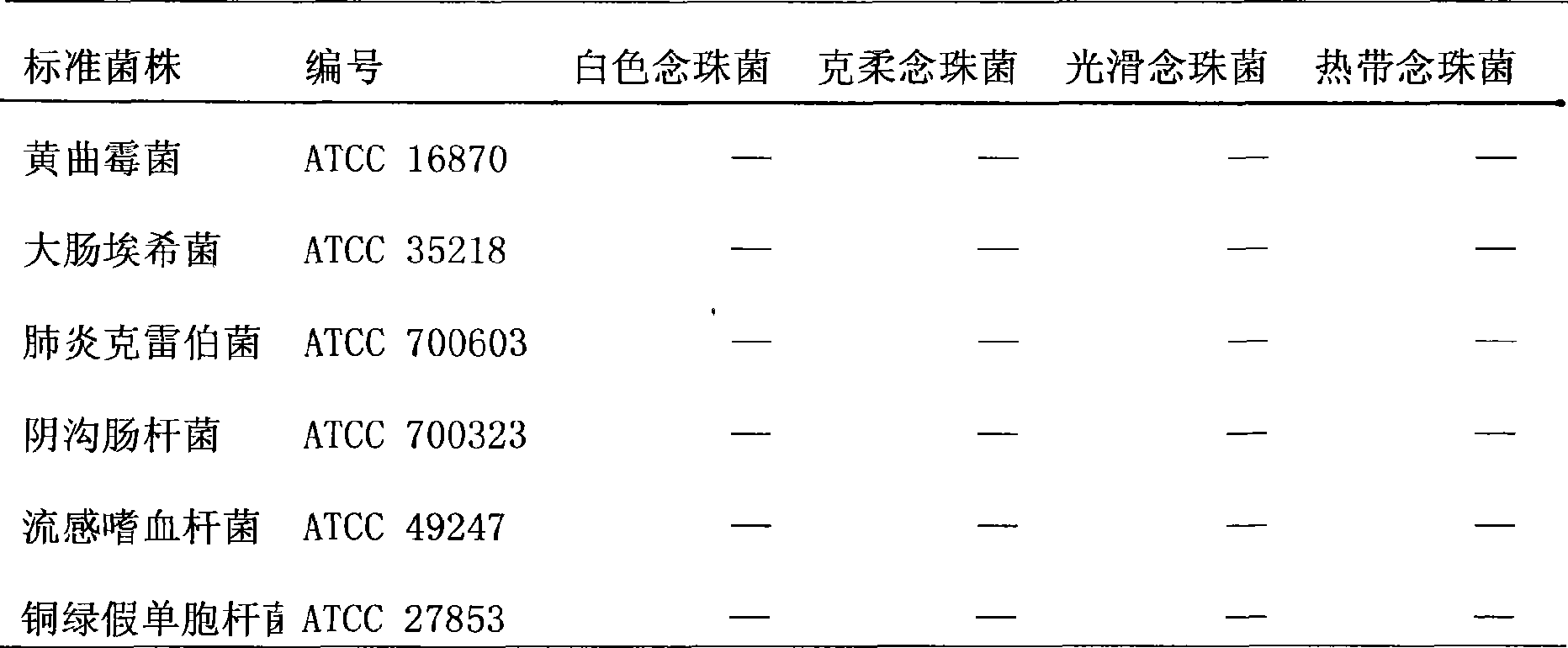
[0052] In conjunction with Table 2 and Table 3, use the kit of the present invention to amplify 13 kinds of 94 clinically common pathogenic fungi, 5 kinds of bacteria and human whole ...
specific Embodiment 3
[0054] Dilute the suspension of Candida albicans, Candida krusei, Candida glabrata, and Candida tropicalis by 10 times: 5×10 8 , 5×10 7 , 5×10 6 , 5×10 5 , 5×10 4 , 5×10 3 , 5×10 2 , 5×10 1 CFU / ml. Fluorescent quantitative PCR amplification was carried out after the DNA was extracted according to the above method, and the result was 5×10 3 The "S" curve can still be observed in CFU / ml, indicating that the fluorescence quantitative PCR method can detect at least 5×10 3 CFU / ml of the above four kinds of Candida, the kit of the present invention has strong sensitivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com