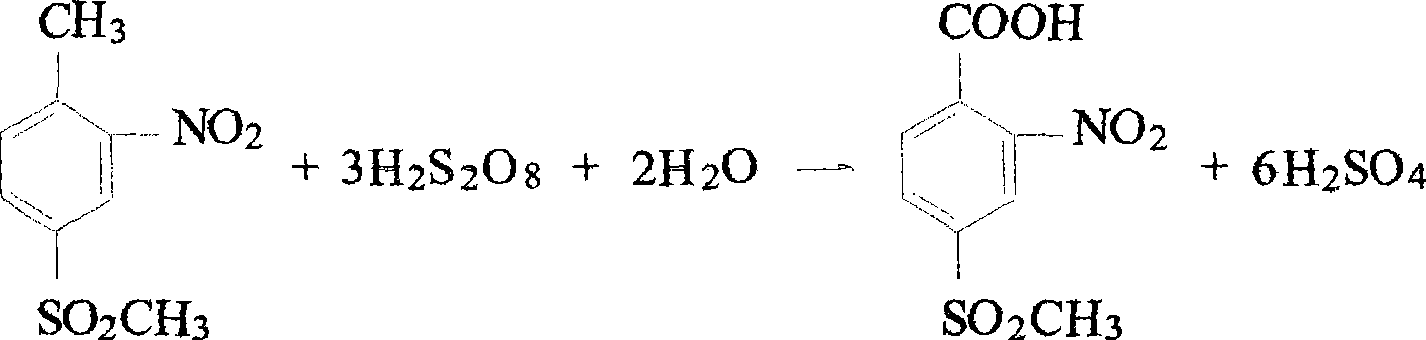Preparation of o-nitro p-methylsulfonylbenzoic acid
A technology of methylsulfonyl benzoic acid and o-nitro group, which is applied in the field of preparation of o-nitro-p-methylsulfonyl benzoic acid, can solve the problems of large amount of hydrogen peroxide, high production cost, easy decomposition and the like, and achieves reduction of waste liquid treatment and recycling , production cost reduction, the effect of cleaner production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0013] The preparation method of this o-nitro-p-methylsulfonyl benzoic acid is to oxidize o-nitro-p-methylsulfone toluene with persulfuric acid to obtain o-nitro-p-methylsulfonyl benzoic acid, and its process is:
[0014] (a) hydrogen peroxide and oleum are mixed at low temperature to prepare persulfuric acid;
[0015] (b) dissolving o-nitro-p-methylsulfone toluene in 70% sulfuric acid;
[0016] (c) adding the persulfuric acid obtained in step a dropwise to the liquid obtained in step b, and the reaction obtains o-nitro-p-methylsulfonyl benzoic acid. After cooling, crystals were precipitated, and the product was obtained by suction filtration.
[0017] In the preparation method of this o-nitro-p-methylsulfonyl benzoic acid, in step a, the concentration of oleum is 20-60% by weight, and the concentration of hydrogen peroxide is 50% by weight; the concentration of sulfuric acid used in step b is 70% weight percent concentration; the amount that each raw material adds in the re...
Embodiment 1
[0028] 1. Preparation of persulfuric acid:
[0029] Place 25.5g of fuming sulfuric acid with a concentration of 60% (weight percent concentration, the same in the future) in a 100mL three-necked round-bottomed flask, under magnetic stirring, slowly add 25.5g of concentration 50% (weight percent concentration, all the same hereafter) H 2 O 2 (0.375mol), the temperature of the ice bath was controlled below 30°C, and the stirring was continued for 15 minutes after the completion of the dropwise addition.
[0030] 2. Preparation of o-nitro-p-methylsulfonyl benzoic acid:
[0031] 5.4g (0.025mol) of o-nitro-p-methylsulfone toluene and 37.6g of 70% H 2 SO 4 The reaction flask was added and the reaction mixture was heated to 80°C. Slowly add 51 g of persulfuric acid prepared in the previous step to the mixed solution dropwise within 4 to 5 hours, control the reaction temperature to 80-100°C during the dropwise addition, cool down to 6°C after the reaction, and obtain ortho-nitrat...
Embodiment 2
[0033] 1. Preparation of persulfuric acid:
[0034] Put 20.0g of 50% oleum in a 100mL three-necked round-bottomed flask, and slowly add 15.3g of 50% H into it dropwise under magnetic stirring. 2 O 2 (0.225mol), the temperature of the ice bath was controlled to be below 30°C, and the stirring was continued for 15 minutes after the dropwise addition, and it was set aside.
[0035] 2. Preparation of o-nitro-p-methylsulfonyl benzoic acid:
[0036] 5.4g (0.025mol) of o-nitro-p-methylsulfone toluene and 37.6g of 70% H 2 SO 4 The reaction flask was added and the reaction mixture was heated to 80°C. 35.3 g of persulfuric acid prepared in the previous step was slowly added dropwise to the mixed solution within 4 to 5 hours, and the reaction temperature was controlled to 80-130°C during the dropwise addition. Nitro-p-methylsulfonylbenzoic acid is 4.3 g of white solid, the yield is 70.0%, and the content by liquid chromatography analysis is 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com