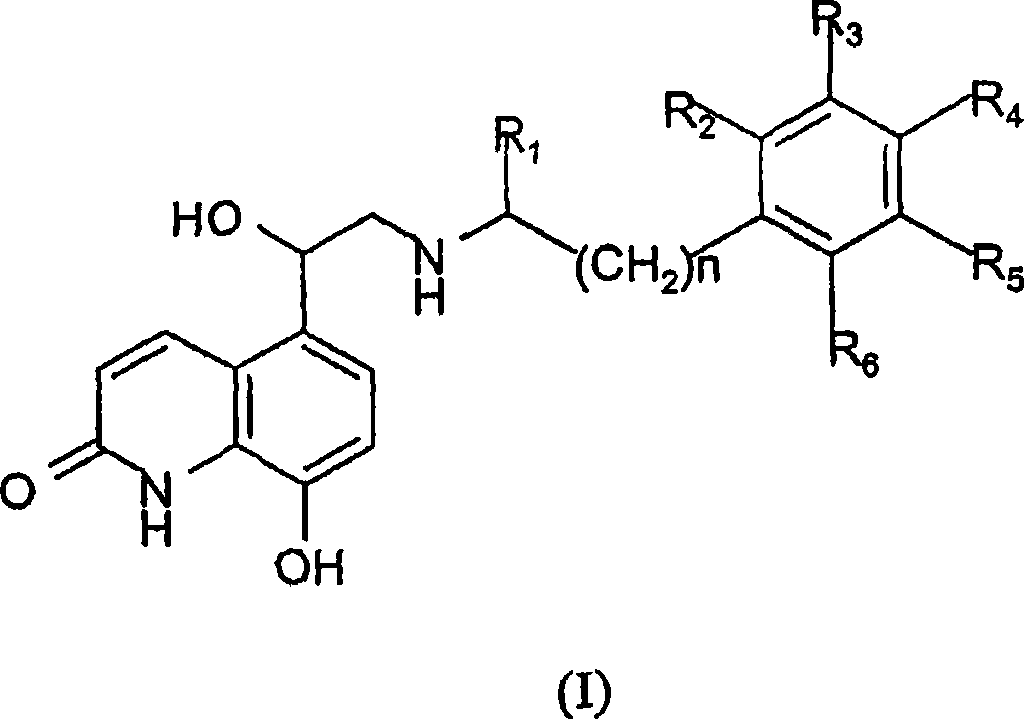
Pressurised metered dose inhalers containing solutions of beta-2 agonists
A pressurized metered dose inhalation and preparation technology, which can be used in medical preparations containing active ingredients, medical preparations without active ingredients, and liquid delivery, etc., and can solve problems such as no therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
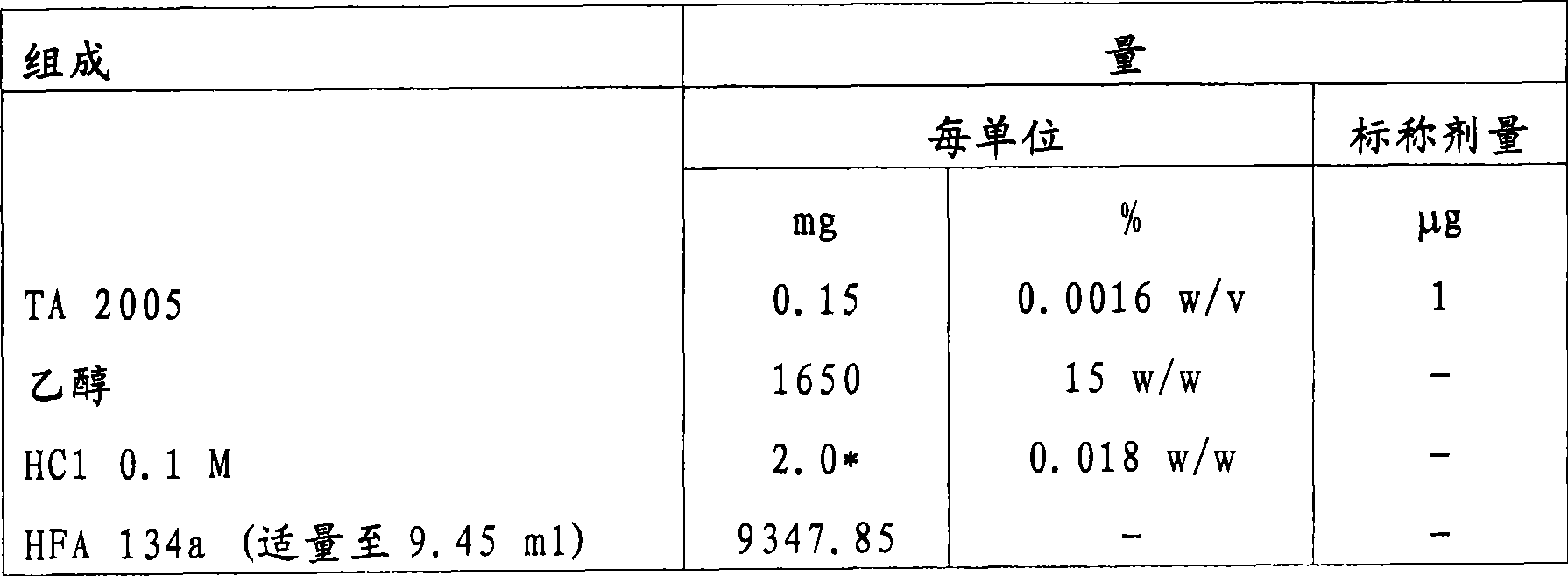
[0092] Example 1 - Ultrafine TA 2005HFA formulation
[0093] A formulation delivering a nominal dose of 1 μg of active ingredient per actuation was prepared with the following composition:
[0094]
[0095] * Equivalent to 2.0μl
[0096] The formulation (120 actuations / jar over 30 actuations) was filled into aluminum cans coated with Teflon on the inner surface (two-stage pressure filling) and equipped with a metering valve with a 63 μl metering chamber. An actuator with a bore diameter of 0.22mm is used. Results were obtained as the average of 2 pots.
[0097] Similarly, formulations may be prepared to deliver nominal doses of 2, 3 or 4 [mu]g of active ingredient per actuation. The aerodynamic particle size distribution was determined by ACI according to page 16, lines 10-18, and the delivery characteristics of each formulation were determined according to the following parameters: i) nominal dose: theoretical dose for a single actuation; ii) delivered dose: Amount of ...
Embodiment 2
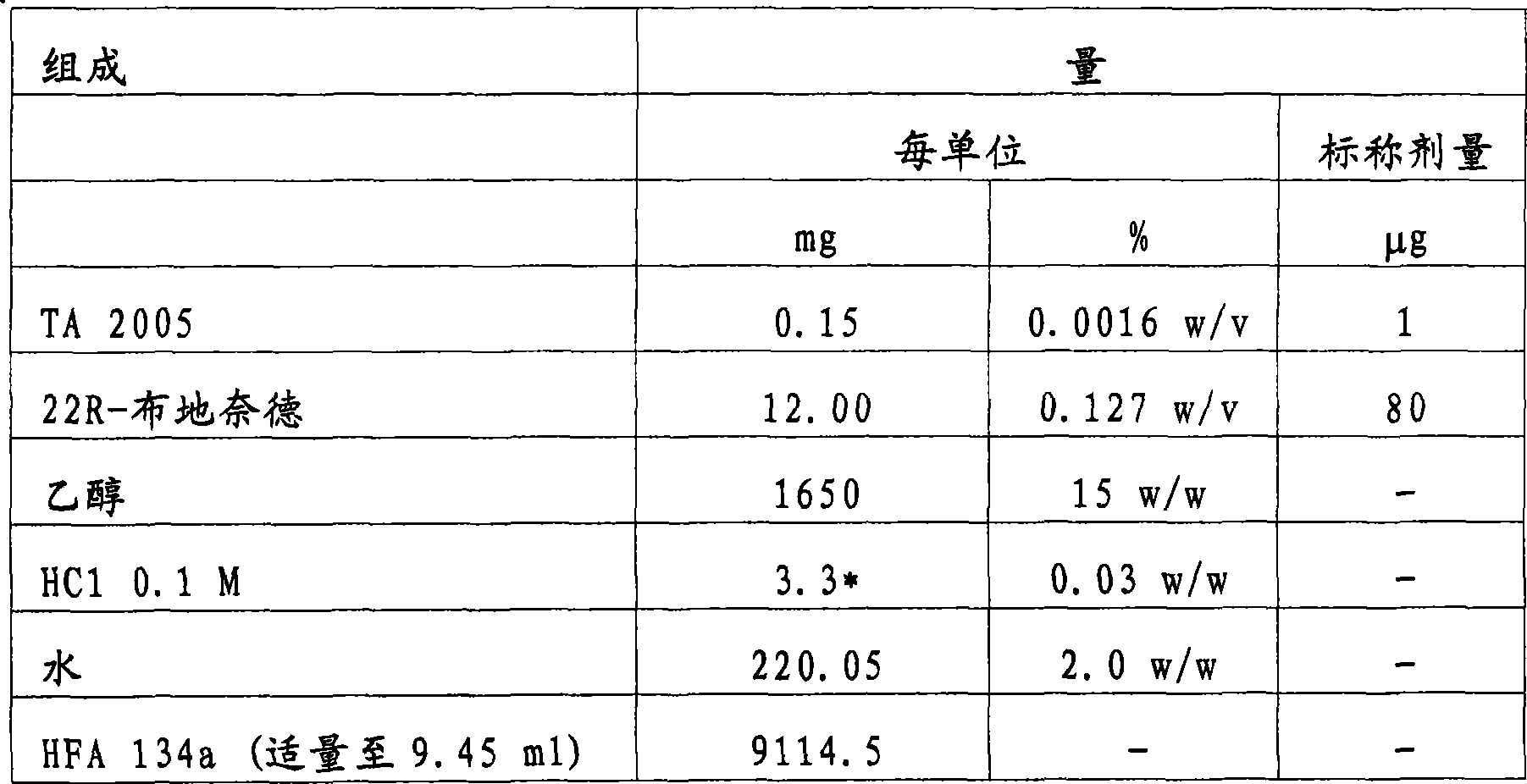
[0102] Example 2 - Ultrafine HFA formulation containing TA 2005 and 22R-budesonide
[0103] A formulation delivering a nominal dose of 1 μg of TA 2005 and 80 μg of 22R-budesonide per actuation was prepared with the following composition:
[0104]
[0105] * Equivalent to 3.3 μl
[0106] The formulation (120 actuations / jar, over 30 actuations) was filled into aluminum cans coated with Teflon on the inner surface (two-stage pressure filling) and equipped with a metering valve with a 63 μl metering chamber.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com