Method for catalytic synthesis of triazine herbicide
A herbicide and catalyst technology, which is applied in the synthesis field of triazine herbicides, can solve the problems of low raw material conversion rate, difficult wastewater treatment, and many side reactions, and achieve simple process flow, good economic and environmental benefits, and improved conversion rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
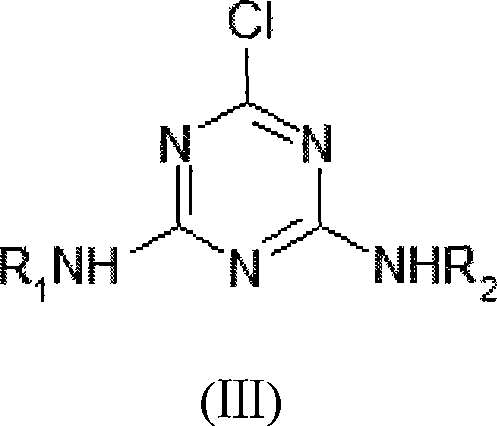
[0046] Synthesis of 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine
[0047](1) In a 500ml four-neck flask, add 200ml of toluene, cool the temperature to -10°C with an ice-salt water bath, and quickly add 50g (0.2711mol) of cyanuric chloride to fully dissolve it; (2) at 5°C Add dropwise 60% isopropylamine solution 26.71g (containing isopropylamine 16.02g, 0.2711mol); after dropwise addition, continue to dropwise add 30% liquid caustic soda solution 37.93g (containing sodium hydroxide 11.38g, 0.2845mol); dropwise Complete, stir 20min; (3) add catalyst tetramethylammonium bromide 0.17g (content 99%, 0.0011mol) in reaction liquid, stir 10min; (4) rise reaction temperature to 25 ℃, dropwise add 70% Monoethylamine solution 17.46g (containing monoethylamine 12.22g, 0.2711mol), continue to dropwise add 30% liquid caustic soda solution 37.93g (containing sodium hydroxide 11.38g, 0.2845mol) after the dropwise addition; 30min; (5) Add 100ml of water to the reaction solution, stir...
Embodiment 2
[0049] Synthesis of 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine
[0050] (1) In a 500ml four-neck flask, add 200ml of toluene, cool the temperature to -5°C with an ice-salt water bath, quickly add 50g (0.2711mol) of cyanuric chloride, stir well, and (2) drop at 0°C Add 26.44g of 60% isopropylamine solution (containing 15.86g of isopropylamine, 0.2684mol); continue to dropwise add 36.15g of 30% liquid caustic soda solution (containing 10.84g of sodium hydroxide, 0.2711mol) after the dropwise addition; , stirred for 20min; (3) added 0.23g of tetraethylammonium bromide (content 99%, 0.0011mol) to the reaction solution, stirred for 10min; (4) raised the reaction temperature to 20°C, and added dropwise 70% of a 17.2 g of amine solution (containing 12.04 g of monoethylamine, 0.2670 mol), after the dropwise addition, 36.15 g of 30% liquid caustic solution (containing 10.84 g of sodium hydroxide, 0.2711 mol) was continued to be added dropwise. After the dropwise addition, s...
Embodiment 3
[0052] Synthesis of 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine
[0053] (1) In a 500ml four-neck flask, add 200ml of toluene, cool the temperature to -2°C with an ice-salt water bath, quickly add 50g (0.2711mol) of cyanuric chloride, and stir evenly; (2) Add dropwise at 5°C 60% isopropylamine solution 26.71g (containing isopropylamine 16.02g, 0.2711mol); after dropwise addition, continue to drip 30% liquid caustic soda solution 36.15g (containing sodium hydroxide 10.84g, 0.2711mol); dropwise, Stir for 20 min; (3) add 0.30 g of benzyltriethylammonium bromide (content 99%, 0.0011 mol) to the reaction solution, and stir for 10 min; (4) raise the reaction temperature to 35° C., and dropwise add 70% of Ethylamine solution 17.46g (containing monoethylamine 12.22g, 0.2711mol), after the dropwise addition, continue to dropwise add 30% liquid caustic soda solution 36.15g (containing sodium hydroxide 10.84g, 0.2711mol); after the dropwise addition, stir for 30min (5) Add 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com