Method for preparing metal complex
A metal complex, metal technology, applied in copper organic compounds, electrolysis process, organic chemistry and other directions, can solve the problems of difficult final separation of products, environmental pollution and other problems, and achieve the effect of convenient separation and purification, broadening research fields and avoiding pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment one, preparation ionic liquid:
[0033](1) Synthesis of 1-butyl-3-methylimidazolium bromide ([Bmim]Br)
[0034] Take N-methylimidazole (6.80mL) and brominated n-butane (10.00mL) with a molar ratio of 1:1.2 into a round bottom flask, put it in a water tank, and irradiate it with microwaves (replace the water in the water tank in time to prevent water becomes hot), stop the radiation after the liquid in the flask turns from mixed to clear, then repeatedly wash with ether several times to remove excess N-methylimidazole, and finally use a vacuum pump to remove Solvent and water gave [Bmim]Br, a colorless ionic liquid. It should be noted that during the reaction process, if the microwave power is too high, the evaporation loss of the halogenated alkanes will increase, and there may be local overheating to decompose the product; while the microwave power is too low, the effect is weak and the reaction cannot be completed; If the medium water temperature is too h...
Embodiment 2
[0039] Embodiment two: complex [Cu 4 (Nmim) 4 (μ 4 -O)(μ-Br) 6 ] of electrochemical synthesis,
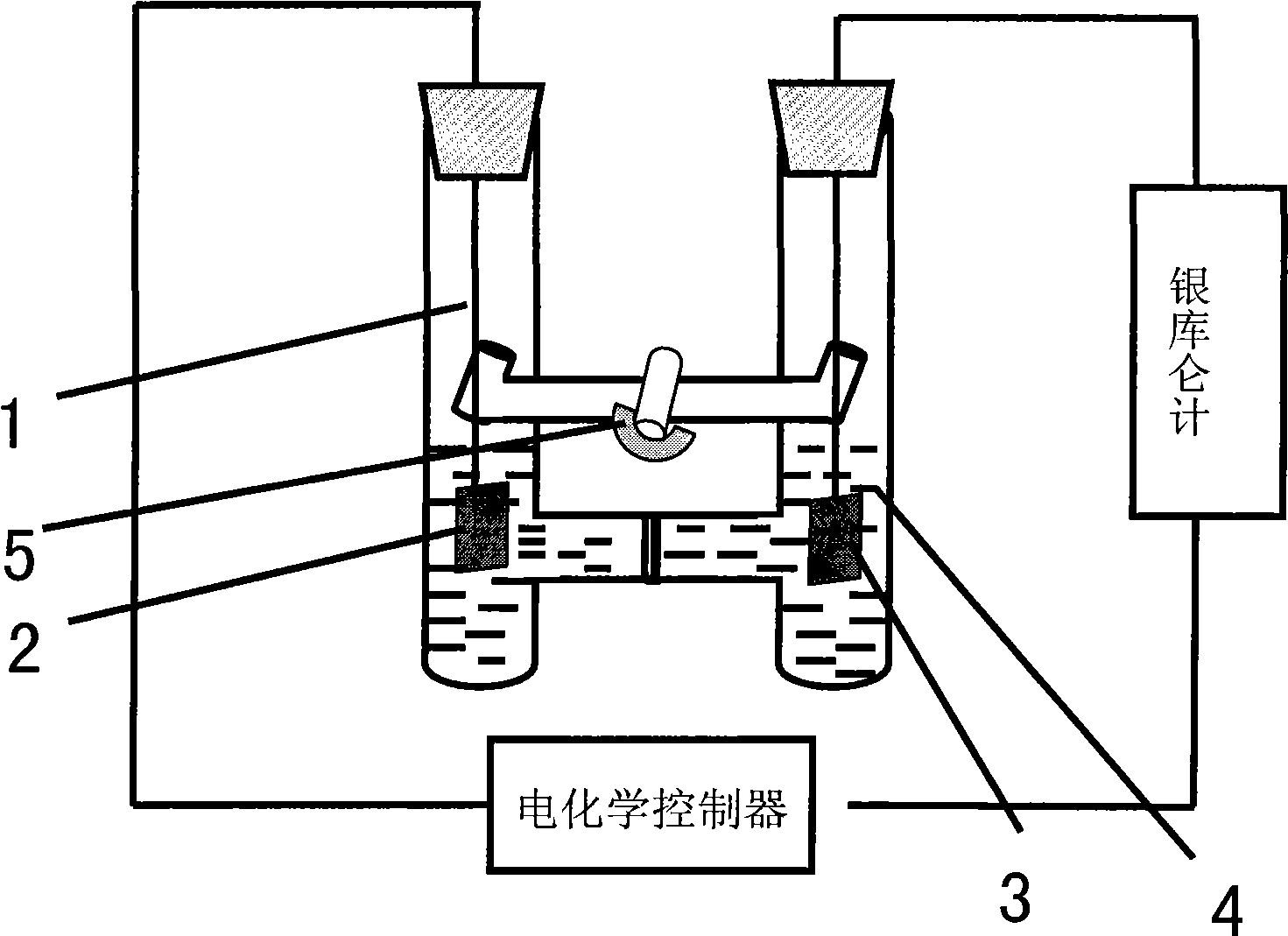
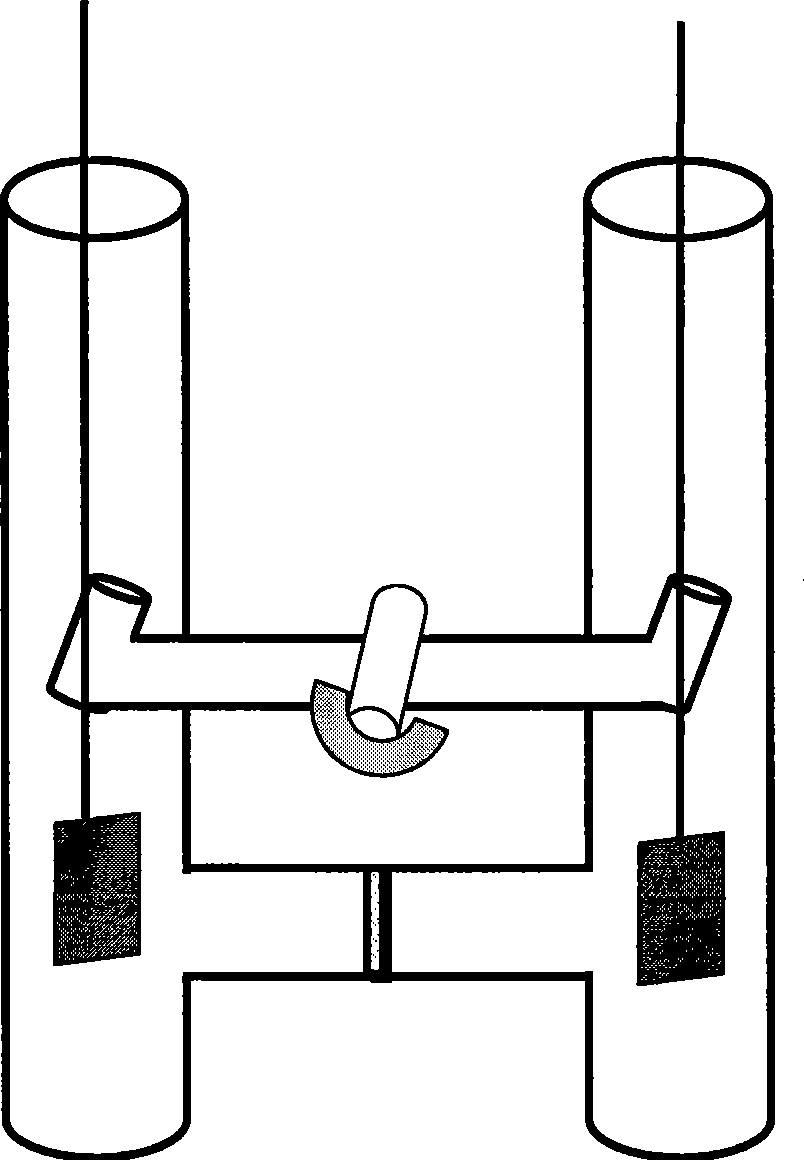
[0040] (1) See attached figure 1 and figure 2 As shown, add N-methylimidazole (1ml) into the anode of the compartment electrolytic cell, stir to make it dissolve in 20mL of ionic liquid [Bmim]Br, add 10mmol ammonium bromide to the cathode of the compartment electrolytic cell, stir to dissolve it In 10mL ionic liquid [Bmim]Br.
[0041] Electrode treatment: Before using the anode metal copper, it is washed with concentrated nitric acid, natural water and distilled water in sequence, then rinsed with acetone, dried and weighed; the metal copper sheet suspended by platinum wire is used as the anode (connected to the electrochemical control instrument) Positive electrode), the platinum sheet suspended by the platinum wire is used as the cathode (connected to the negative electrode of the electrochemical control instrument), and the two platinum wires are respectively passed throu...
Embodiment 3
[0045] Embodiment three: complex [Cu(Nmim) 4 Br]Br H 2 Electrochemical synthesis of O,
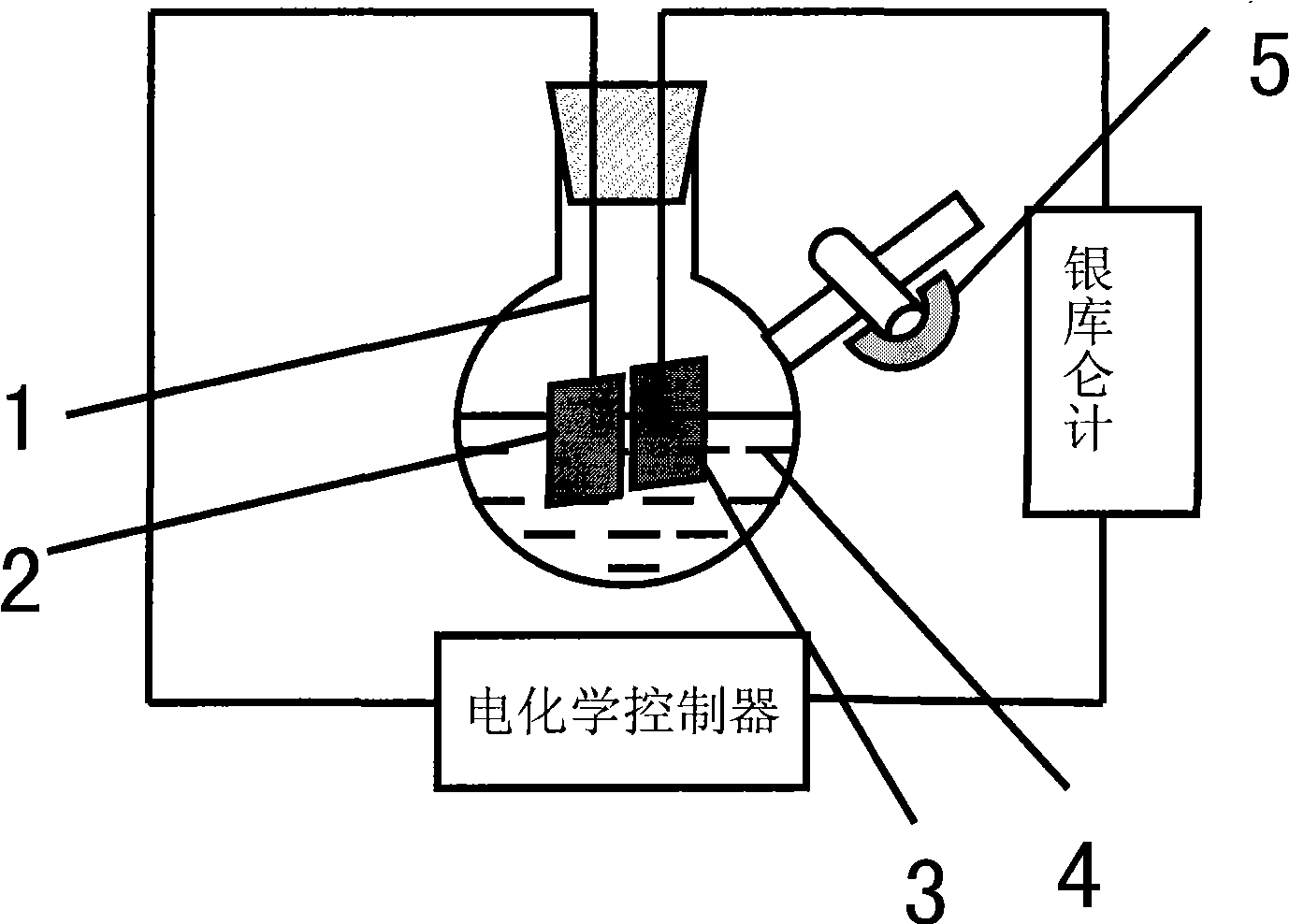
[0046] (1) if image 3 As shown, add 10mL ionic liquid [Bmim]Br into a 100mL Schlenk flask, add 1ml N-methylimidazole to the above system, stir to dissolve it in the ionic liquid [Bmim]Br;
[0047] The metal copper sheet suspended by the platinum wire is used as the anode (connected to the positive electrode of the electrochemical control instrument), and the platinum sheet is used as the cathode (connected to the negative electrode of the electrochemical control instrument). The current of the reaction process is provided by the electrochemical controller. The total electricity is measured by a silver coulomb meter connected in series in the circuit.
[0048] See attached Figure 4 As shown, the electrolytic cell was evacuated for one minute, then filled with inert gas Ar gas, and this operation was repeated three times to ensure that the reaction system was under inert atmosphere con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com