Methods for reducing viral load in hiv-1-infected patients
An HIV-1, virus technology, applied in chemical instruments and methods, botanical equipment and methods, applications, etc., can solve problems such as untested CCR5 coreceptor inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
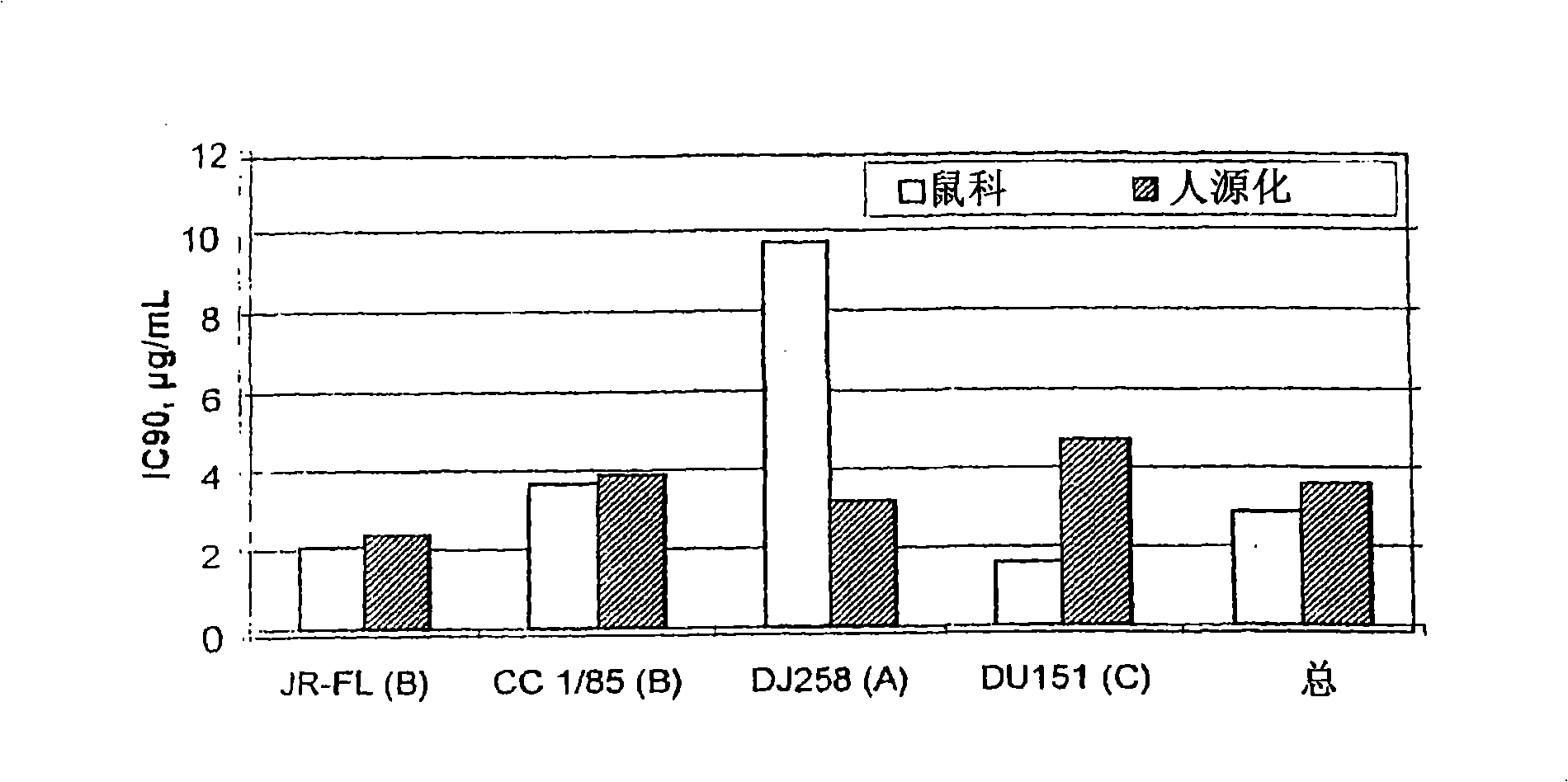
[0087]The present invention provides a method for reducing HIV-1 viral load in a human subject infected with HIV-1, which comprises administering an effective HIV-1 viral load reducing dose to said subject at predetermined time intervals ( a) Humanized antibody PRO 140, or (b) anti-CCR5 receptor monoclonal antibody, said humanized antibody PRO 140 or anti-CCR5 receptor monoclonal antibody (i) and CD4+ in the subject CCR5+ cells bind and inhibit the fusion of HIV-1 and the cells, (ii) inhibit the fusion of HIV-1 and CD4+CCR5+ cells, the efficacy is equal to or higher than that of PRO140, (iii) without reducing the body of the subject Under the condition of the number of CD4+CCR5+ cells, covering the subject’s CD4+CCR5+ cells, and / or (iv) without inducing an increase in the plasma concentration of the subject’s circulating beta chemokine, and the subject’s CD4+CCR5+ cell combination, where PRO140 includes (i) two light chains, each light chain contains the expression product of plas...
Embodiment 1
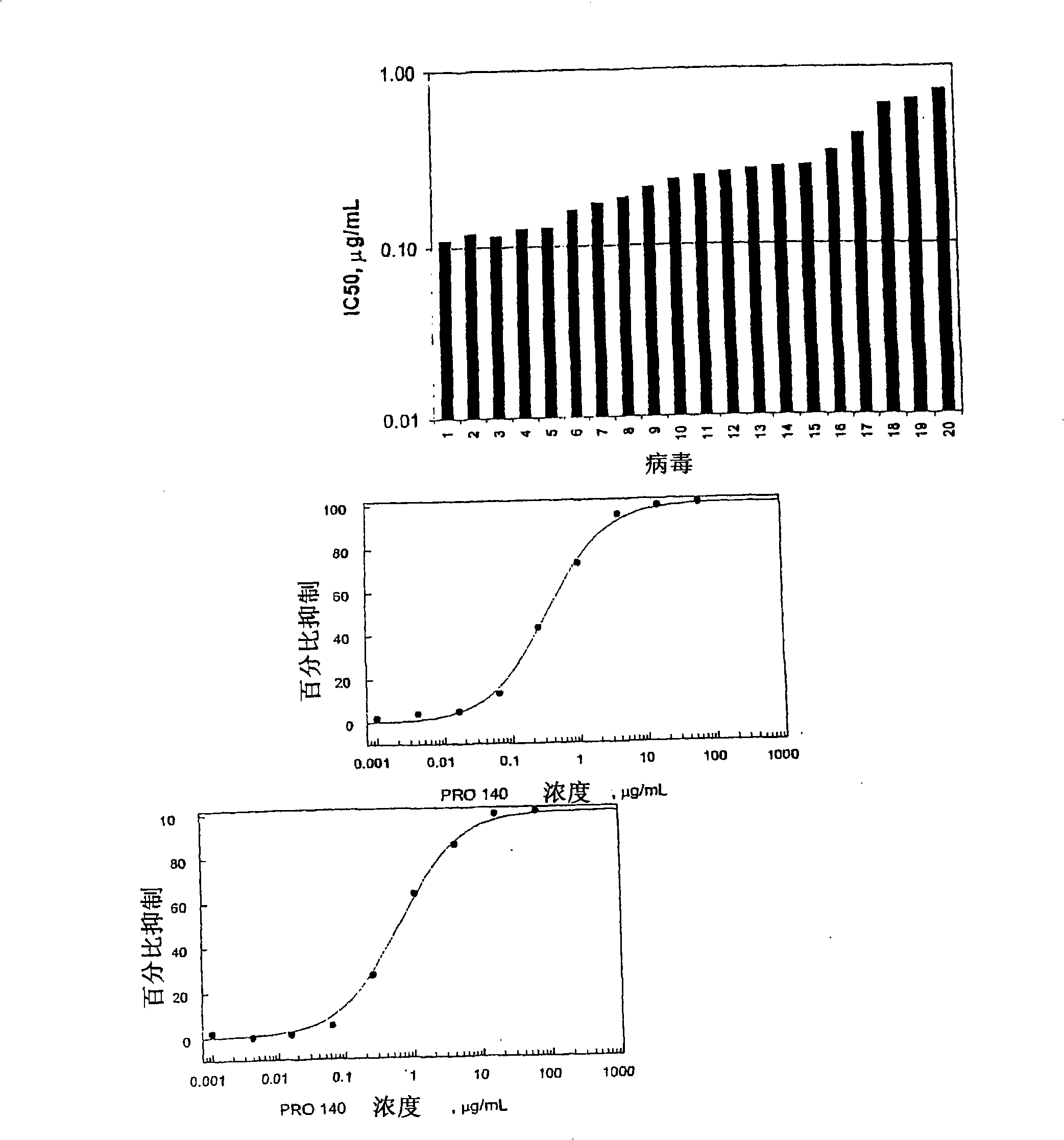
[0210] Example 1: Combination testing of PRO 140 and HIV-1 invasion inhibitor in a fluorescent RET assay
[0211] Materials and methods
[0212] Compound and mAb
[0213] PRO 140 was prepared by expressing in Sp2 / 0 cells using hybridoma serum-free medium (Invitrogen, Carlsbad, CA) supplemented with 2mM L-glutamine. A 5.0 μm Depth filter (Sartorius, Goettingen, Germany) was used to purify a large amount of mAb, and then passed through a 0.2 μm sterilization grade filter (Sartorius). The mAb was purified by passing through an affinity column (MabSelect Protein A column, Amersham, Piscataway, NJ) and then by ion exchange chromatography (SP Sepharose Cation Exchange Resin, Amersham). Use Viresolve TM 10 Opticap NFP capsules (Millipore, Billerica, MA) followed by 0.2 μm filter nanofiltration PRO 140, and concentrated / diafiltered PRO 140 through a disposable TFF box (Millipore). Then, the mAb was purified through a hydroxyapatite column (Bio-Rad, Hercules, CA), concentrated to 10 mg...
Embodiment 2
[0298] Example 2: In the determination of HIV-1 pseudoviral particles (HIV-1PP), PRO 140 and small molecules, peptides and Protein inhibitor, combined test with HIV-1
[0299] Materials and methods
[0300] Preparation of HIV-1 fake particles
[0301] Through HIV-1 based NL4 / 3luc+env-plasmid and encoding HIV-1 JRFL Transient co-expression of Env constructs produced HIV-1 pseudoparticles (HIV-1pp) in 293T cells. NL4 / 3luc+env-plasmid was obtained from NIH AIDS research and reference reagent program (Cat.No.3418), and HIV-1 JRFL Env was inserted into the pcDNA3.1 vector (Invitrogen). Briefly, 293T cells were transfected with NL4 / 3luc+env-receptor vector and Env expression vector (Profection Mammalian Transfection Kit, Promega) calcium phosphate in a ratio of 1:1 in Hepes buffer. After 16 hours of transfection, the medium was aspirated, and fresh cell culture medium (DMEM containing 10% FBS, glutamine and antibiotics) was added, and the culture was continued at 37°C for another ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com