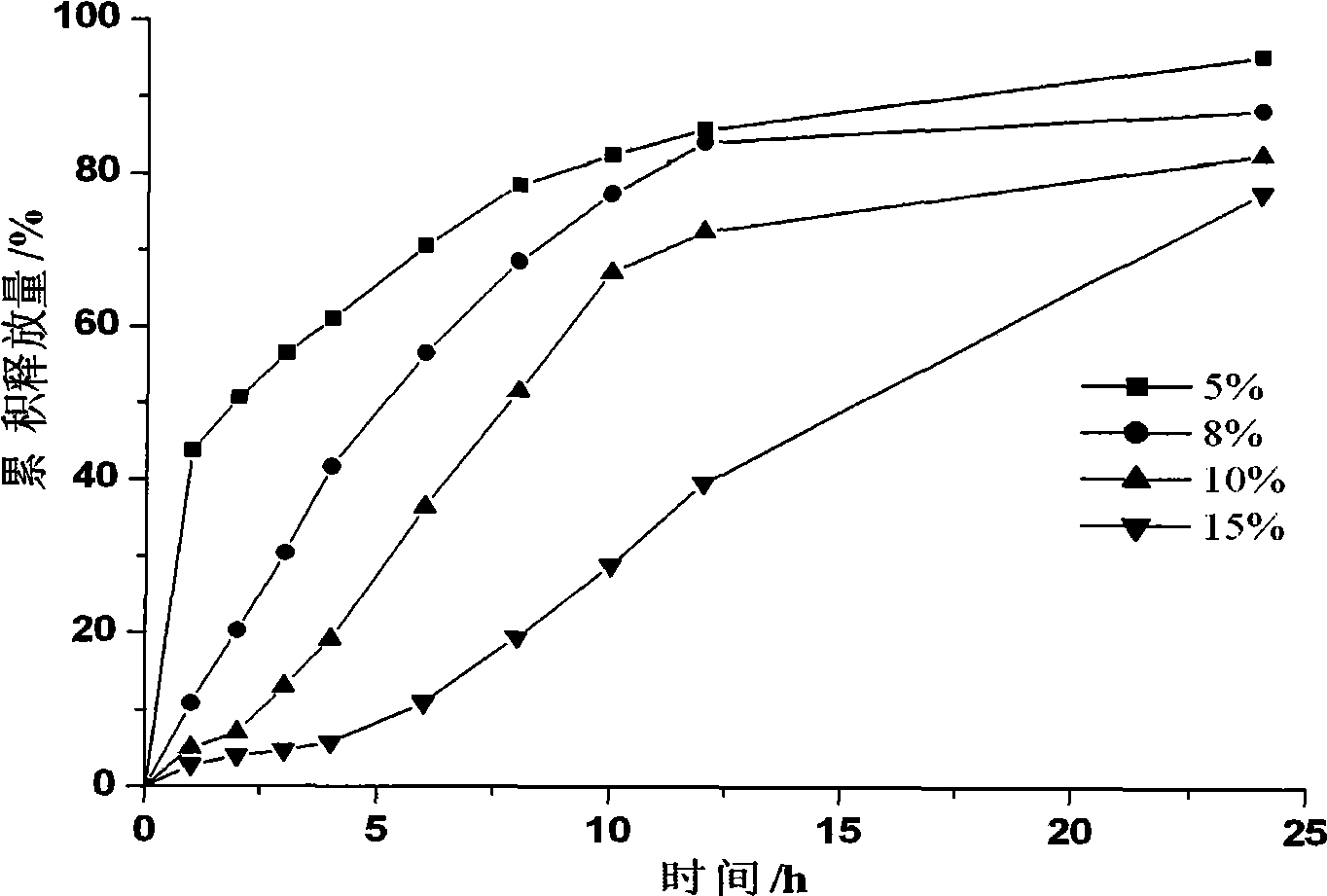
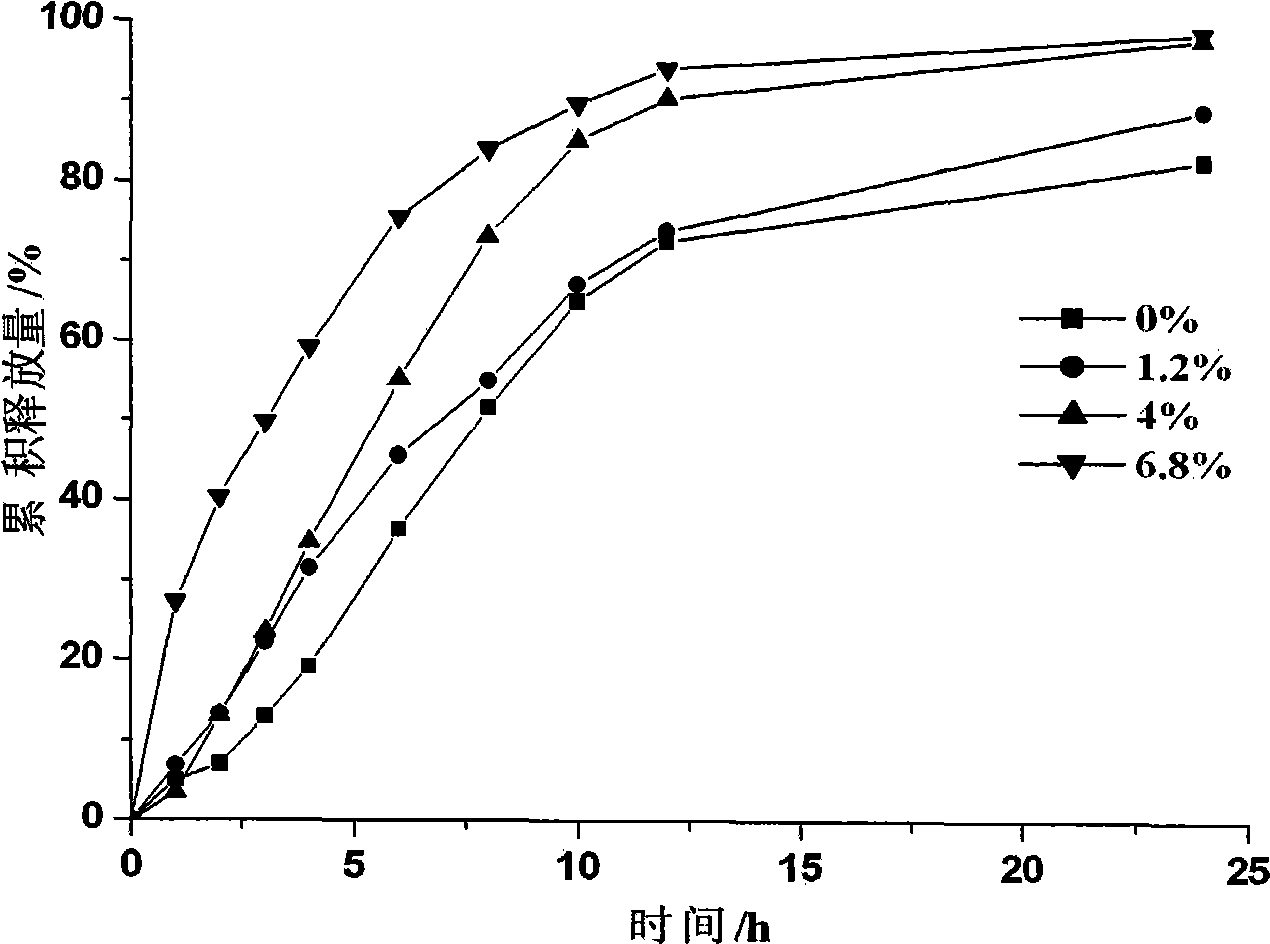
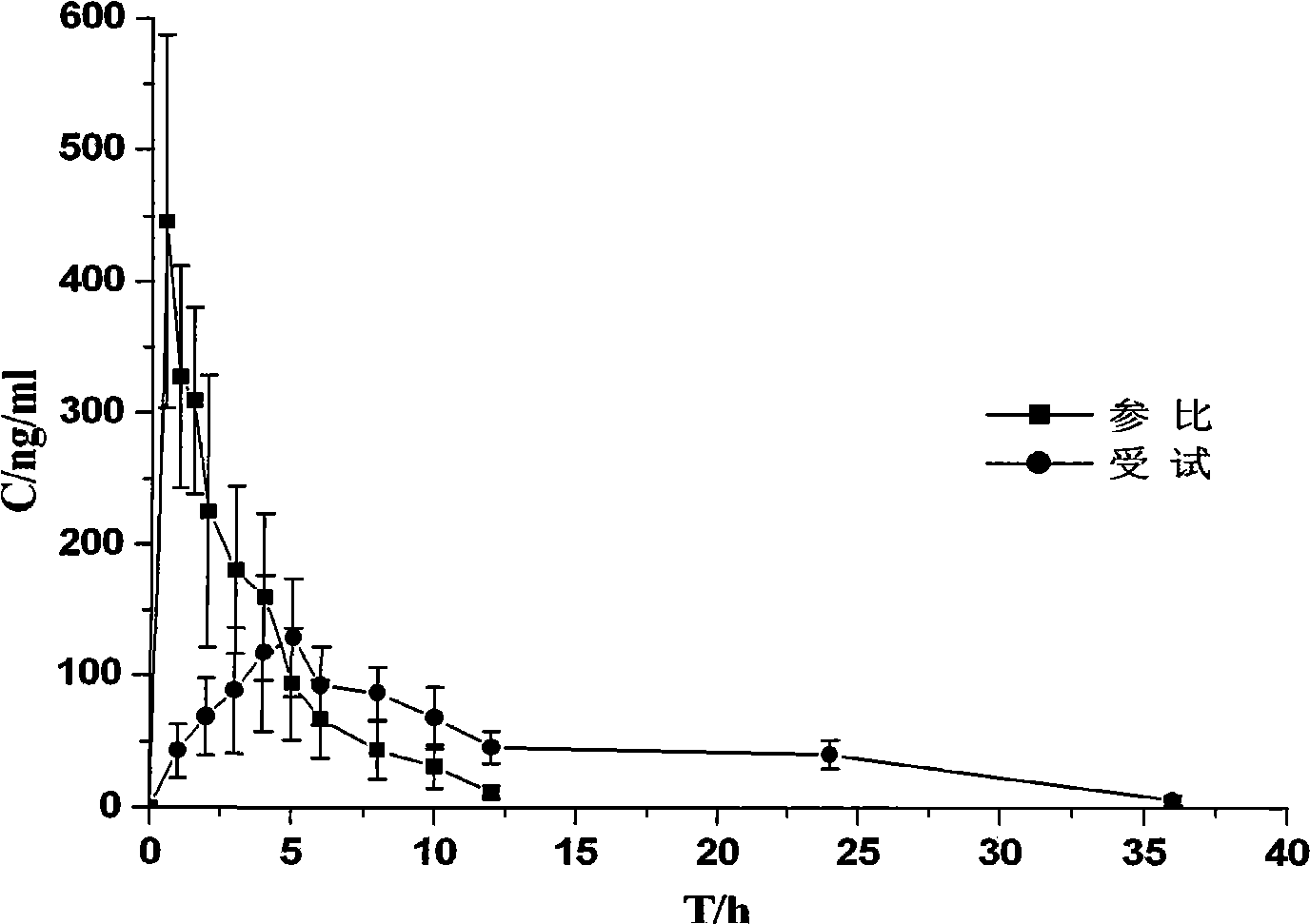
Bupropion hydrochloride sustained-release pellet and preparation method thereof
A technology of bupropion hydrochloride and sustained-release pellets, which is applied in the direction of non-active ingredient medical preparations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the problems of release differences, reduce production costs, High solubility, no pH-dependent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Step 1: Preparation of Pill Hearts Containing Pills
[0041] prescription:
[0042] Bupropion Hydrochloride 300g
[0043] Microcrystalline Cellulose 180g
[0044] Lactose 20g
[0045] Distilled water 250g
[0046] Preparation process: Grind bupropion hydrochloride raw material, pass through 80-mesh sieve with microcrystalline cellulose and lactose respectively, then sieve and mix three times, add distilled water to make softener, and extrude the softener into bars at an extrusion speed of 20rpm Then put it into a spheronizer and spheronize at a speed of 20HZ for 8min. Afterwards, the pill core is dried overnight at 50°C, and the 20-40 mesh pellets are taken for later use.
[0047] Step 2: Pack the drug-containing pill core in step 1 with a Surelease coating.
[0048] prescription:
[0049] Surelease (solid content 25%) 240g
[0050] Distilled water 510g
[0051] PEG6000 2.4g
[0052] Preparation process: Dilute Surelease with distilled water, then add PEG6000 t...
Embodiment 2
[0055] Step 1: Preparation of Pill Hearts Containing Pills
[0056] prescription:
[0057] Bupropion Hydrochloride 300g
[0058] Microcrystalline Cellulose 116g
[0059] Lactose 13g
[0060] Distilled water 160g
[0061] Preparation process: Grind bupropion hydrochloride raw material and pass through 80-mesh sieve with microcrystalline cellulose respectively, then sieve and mix three times, add distilled water to make softener, and extrude the softener into strips at an extrusion speed of 20rpm , and then put it into a spheronizer and spheronize at a speed of 20HZ for 8 minutes, then dry the core of the pills loaded at 50°C overnight, and take 20-40 mesh pellets for later use.
[0062] Step 2: Coat the pill core in step 1 with a Surelease coating.
[0063] prescription:
[0064] Surelease (solid content 25%) 172g
[0065] Distilled water 364g
[0066] Lactose 0.514g
[0067] Preparation process: Dilute Surelease with distilled water, then add lactose and magnetically ...
Embodiment 3
[0070] Step 1: Preparation of Pill Hearts Containing Pills
[0071] prescription:
[0072] Bupropion Hydrochloride 300g
[0073] Microcrystalline Cellulose 200g
[0074] Distilled water 250g
[0075] Preparation process: Grind bupropion hydrochloride raw material and pass through 80-mesh sieve with microcrystalline cellulose respectively, then sieve and mix three times, add distilled water to make softener, and extrude the softener into strips at an extrusion speed of 20rpm , and then put it into a spheronizer and spheronize at a speed of 20HZ for 8 minutes, then dry the core of the pills loaded at 50°C overnight, and take 20-40 mesh pellets for later use.
[0076] Step 2 Coat the core of the drug-containing pill in step 1 with HPMC.
[0077] prescription:
[0078] HPMC 15g
[0079] PEG6000 1.5g
[0080] Distilled water 285g
[0081] Preparation process: Dissolve 15gHPMC (viscosity 5cps) and 1.5gPEG6000 in distilled water, place the pill core in the material hopper of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com