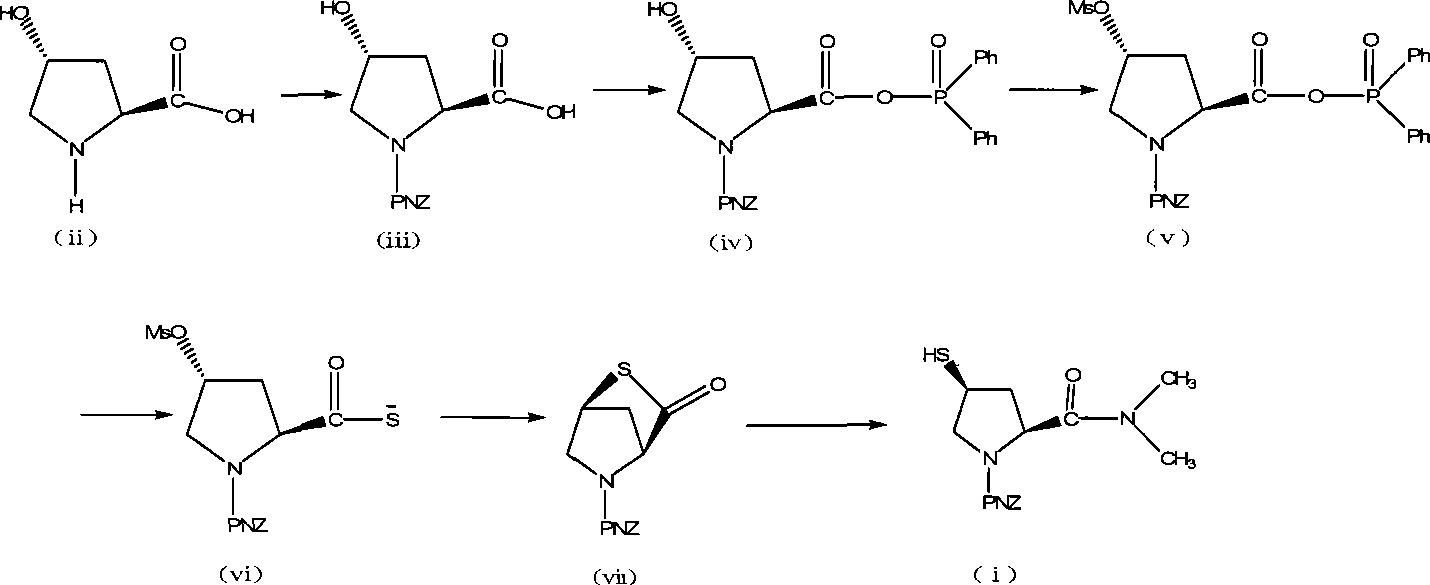
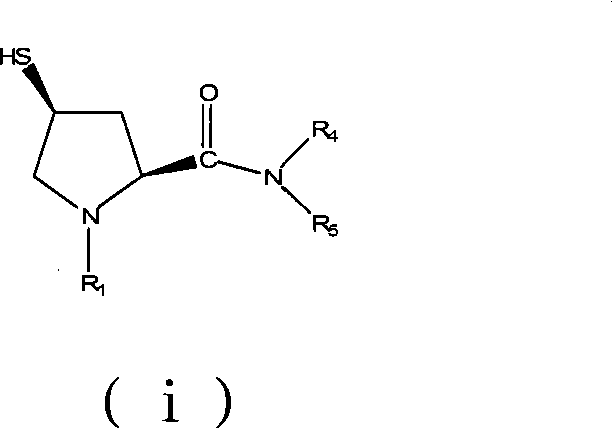
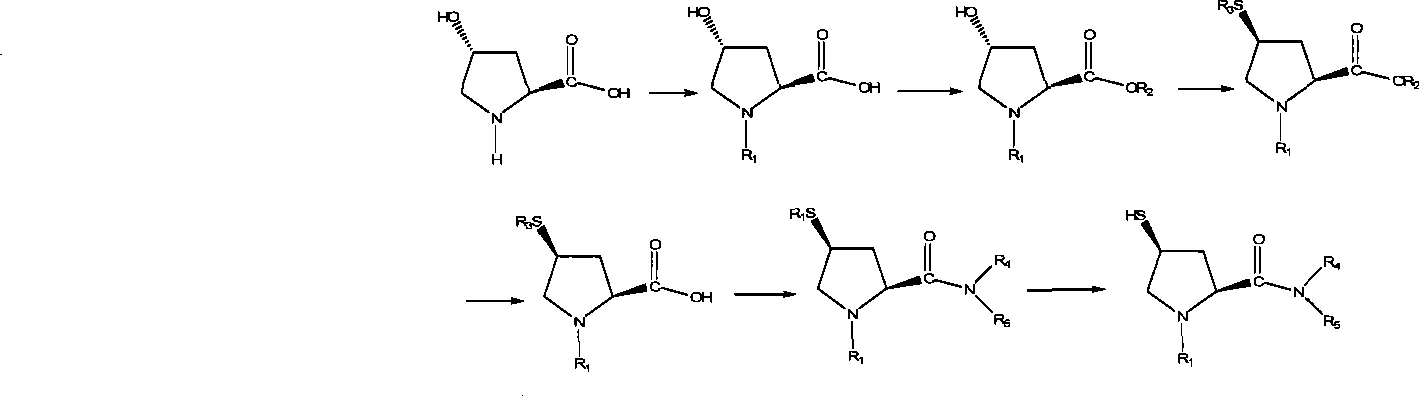
Preparation of carbapenem antibiotic side chain
A carbapenem and antibiotic technology, applied in the field of medicine, can solve the problems of high cost and complicated preparation process, and achieve the effects of simplified operation, easy purification and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, preparation of trans-1-(p-nitrobenzyloxycarbonyl)-4-hydroxyl-L-proline compound (iii)
[0037] Add 100g (0.763mol) of L-hydroxyproline and 500ml of water, stir to dissolve, cool to 0°C, add a solution of p-nitrobenzyl chloroformate (181g, 0.84mol) in dichloromethane (268.6ml), dropwise Potassium carbonate (133.9 g, 0.968 mol) in water (268.6 ml) was dripped in 1.5 hours, kept for 2 hours, and then reacted at room temperature for 2 hours. Separate the dichloromethane phase, wash the water phase with dichloromethane (200ml×1), add concentrated hydrochloric acid to adjust the pH to 1-2, stir overnight, filter with suction after the solid precipitates, wash with water, and dry at 50°C to obtain trans- 1-(p-nitrobenzyloxycarbonyl)-4-hydroxy-L-proline (iii) crude product m=235.6g. The yield is 99.61%.
Embodiment 2
[0038] Embodiment 2, preparation of trans-1-(p-nitrobenzyloxycarbonyl)-4-hydroxyl-L-proline compound (iii)
[0039] Add 100g (0.763mol) of L-hydroxyproline and 500ml of water, stir to dissolve, cool to 0°C, add a solution of p-nitrobenzyl chloroformate (181g, 0.84mol) in dichloromethane (268.6ml), dropwise A solution of sodium hydroxide (67.2g, 1.68mol) in water (268.6ml) was dropped in 1.5 hours, kept for 2 hours, and then reacted at room temperature for 2 hours. Separate the dichloromethane phase, wash the water phase with dichloromethane (200ml×1), add concentrated hydrochloric acid to adjust the pH to 1-2, stir overnight, filter with suction after the solid precipitates, wash with water, and dry at 50°C to obtain trans- 1-(p-nitrobenzyloxycarbonyl)-4-hydroxy-L-proline (iii) crude product m=236g. The yield is 99.78%.
Embodiment 3
[0040] Example 3, purification of trans-1-(p-nitrobenzyloxycarbonyl)-4-hydroxyl-L-proline compound (iii)
[0041] After dissolving 235.6 g of trans-1-(p-nitrobenzyloxycarbonyl)-4-hydroxy-L-proline (iii) crude product under reflux with 668 ml of ethyl acetate, the temperature was lowered to 75°C, and 0.5 g of activated carbon was added. Suction filtration, the filtrate was crystallized by cooling at 0°C, suction filtration, the solids were washed with ethyl acetate, the ethyl acetate phases were combined, and rotary evaporated at 40°C, the solids were precipitated, suction filtration, washed with ethyl acetate, the solids were combined, and dried at 40°C , to obtain trans-1-(p-nitrobenzyloxycarbonyl)-4-hydroxy-L-proline (iii) refined product 213.1 g, yield 90.1%.
[0042] The physical properties of trans-1-(p-nitrobenzyloxycarbonyl)-4-hydroxy-L-proline (iii) refined product are as follows
[0043] Molecular formula: C 13 h 14 N 2 o 7 ;
[0044] Molecular weight: 310;
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com