Recombinant plasmid and construction method thereof
A recombinant plasmid and plasmid technology, applied in recombinant DNA technology, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of low expression and low solubility of isocitrate dehydrogenase, and achieve the expression and purification process. Simple, reduce production costs, and save resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
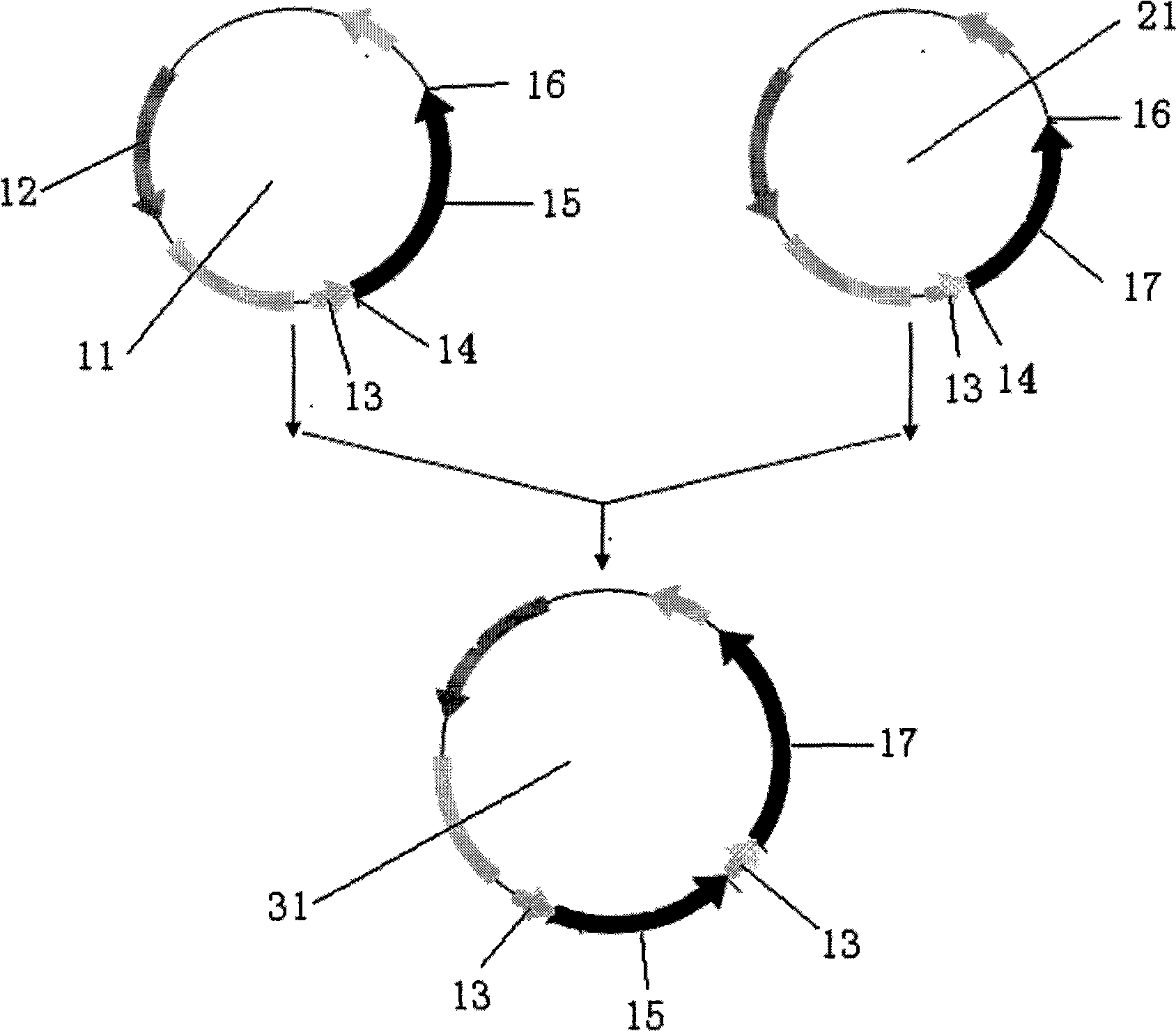
[0030] 1. Construction of pUX plasmid
[0031] Using the genome of E.coli MG1655 as a template, design upstream and downstream primers:
[0032] Upstream primer: 5′-CGCAGCACTCTAGA CATGCCACATTCCTACGATT-3′;
[0033] Downstream primer: 5′-GCCAAACACTCGAG TTAAAACAGGCGGTTTAAACCGTTTAAC-3
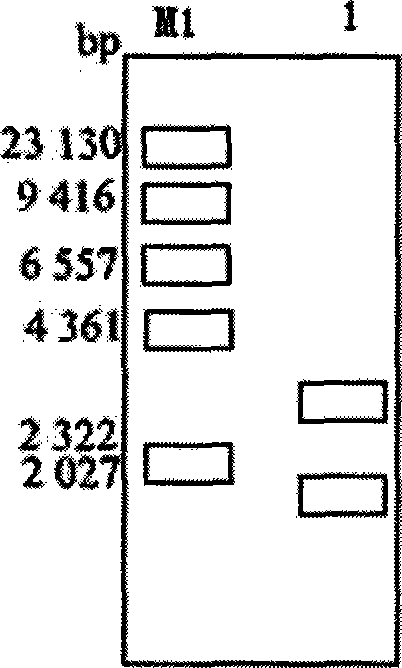
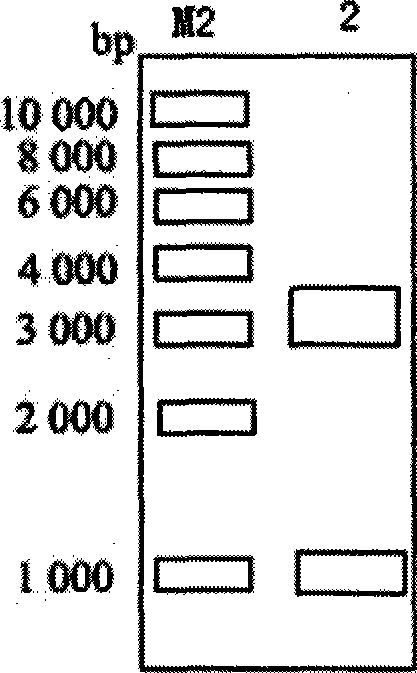
[0034] Perform PCR amplification, and the reaction conditions are: 94°C for 3min; 94°C for 30s; 55°C for 40s; 72°C for 1.5min; 35 cycles; 72°C for 10min. Respectively use Xba I and Xho I to double-enzyme digest the PCR product, recover a linear fragment of about 1.4kb, insert it into the plasmid pBluescript II SK(+) digested with Xba I and Xho I, transform DH5α, Amp screens the single clone, and performs enzyme Excision identification and sequencing identification to obtain pUX.
[0035] like figure 2 Shown: The size of the recombinant plasmid is about 4.3kb. Since the udhA gene is inserted into the plasmid pBluescript II SK(+) after double digestion with Xba I and XhoI, after double digestion...
Embodiment 2
[0047] Example 2: Expression of SmIDH
[0048] The pSX and pUS strains prepared in Example 1 were induced by isopropyl-β-D-thiogalactopyranoside (IPTG), and polyacrylamide gel electrophoresis (SDS-PAGE electrophoresis) showed that: as a control, the The specific band of SmIDH (about 44ku) expressed by the recombinant strain of plasmid pSX was not obvious, which was almost the same as that of the strain containing pBluescript II SK (+) blank plasmid; the recombinant strain containing plasmid pUS could express two obvious specific bands The molecular weights of UdhA (about 53ku) and SmIDH (about 44ku) were basically consistent, indicating that both UdhA and SmIDH were effectively expressed in the double-promoter and double-gene expression vector system.
Embodiment 3
[0049] Example 3: SmIDH activity staining
[0050] The pSX and pUS prepared in Example 1 were transformed into Escherichia coli for expression, and the prepared crude enzyme solution was subjected to non-denaturing gel electrophoresis. + or NADP + Carry out the catalyzed reaction of isocitrate dehydrogenase in the staining buffer solution, and develop the color in a 37°C incubator for 1-2h. IDH activity staining experiments showed that: SmIDH expressed by pUS and pSX can use NAD + Catalyze the reaction as a coenzyme, but not with NADP + The reaction was catalyzed by the coenzyme, and the crude enzyme solution of SmIDH expressed by pUS showed thicker staining bands under the same conditions, reflecting the higher expression level of pUS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com