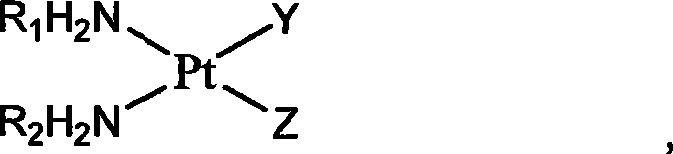Platinum complexes
A technology for platinum complexes and pharmaceutical preparations, applied in the field of platinum complexes and their preparation, can solve the problems of large side effects, low water solubility, short remission period and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: Synthesis of cis-diiodo (1R, 2R)-1,2-trans cyclohexanediamine platinum
[0065] Add 100ml of water to dissolve 4.2g of potassium chloroplatinite, add 16.6g of potassium iodide in 20ml of aqueous solution, and stir at room temperature for 10 minutes in the dark under nitrogen, then add 1.1g of (1R,2R)-1,2-trans-cyclohexanediamine 5ml of aqueous solution, stirred at room temperature for 45 minutes, filtered, washed with water, ethanol, ether, and dried in vacuum at 40°C. Weighed 5.3g, yield: 94.1%.
Embodiment 2
[0066] Embodiment 2: cis-diiodoethylenediamine platinum synthesis
[0067] Dissolve 2g of potassium chloroplatinite in 40ml of water, add 4g of potassium iodide in 5ml of aqueous solution, pass nitrogen to avoid light and react at room temperature for 10 minutes, add dropwise 0.4g of ethylenediamine in 5ml of aqueous solution, after the dropwise addition, continue stirring for 4 hours, filter, The filter cake was washed with water, ethanol and ether, and dried under vacuum at 40°C. Weighed 1.9g, yield: 78.0%.
Embodiment 3
[0068] Embodiment 3: cis-diiodine diammine platinum is synthesized
[0069] Dissolve 2.1g of potassium chloroplatinite in 50ml of water, add 8.3g of potassium iodide in 10ml of aqueous solution under dark conditions, stir for 10 minutes, add ammonia water (25%-28%) dropwise, keep the pH at about 8, and react for 5 hours After filtering, the filter cake was washed with water, ethanol and ether, and dried under vacuum at 40°C. Weighing: 2.25g, yield: 93.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com