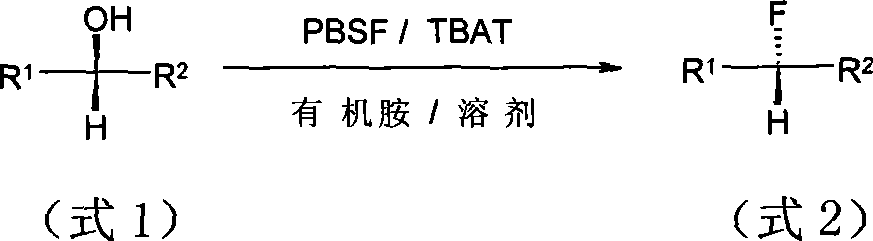
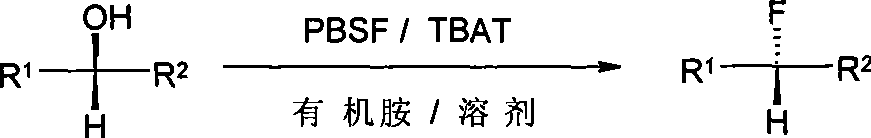
Method for synthesizing alcoholic hydroxyl fluorine derivatives
The technology of a hydroxyl derivative and a synthesis method is applied in the synthesis field of fluorination of alcohol hydroxyl group, which can solve the problems of difficult preparation, strong corrosion, high price and the like, and achieve the effects of eliminating by-products, reducing impurities and reducing by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0042] Add 9-benzoyloxy-1-nonanol (3.10g, 11.7mmol), TBAT (5.04g, 93.6mmol), diisopropylethylamine (4.82ml, 3.77g, 29.3mmol) in sequence at room temperature And 56ml of toluene. After stirring for several minutes, PBSF (7.77g, 25.7mmol) was added, and the reaction was stirred for 24h, and the reaction was followed by TLC. After the reaction was completed, it was concentrated by rotary evaporation, and the residue was subjected to column chromatography to obtain 2.93 g of a colorless oil with a yield of 93.9%. R f =0.38 (petroleum ether:acetone=160:1).IR(NaCl): 3071,2932,2857,1720,1275,1123,714cm -1 ; MSm / z: 267[M+1] + ; 1 H NMR(CDCl 3 )δ=1.27~1.48(m, 11H), 1.63~1.81(m, 4H), 4.32(t, J=5.3Hz, 2H), 4.44(dt, J=39.5 / 5.0Hz, 2H), 7.34~7.58 (m, 3H), 7.65 (d, J = 6.0 Hz, 1H), 8.05 (d, J = 6.0 Hz, 1H).
Embodiment 3
[0044] Add (2S, 4R)-N-[4,4-bi(3-methyl-2-thienyl)-3-butenyl]-4-hydroxypyrrolidine-2-carboxylic acid methyl ester one by one at room temperature (3.87g, 10.0mmol), TBAT (4.32g, 8.00mmol), diisopropylethylamine (4.16ml, 3.24g, 25.0mmol) and 47ml of toluene. After stirring for a few minutes, add PBSF (6.64g, 22.0mmol) , The reaction was stirred for 24h, and the reaction was followed by TLC. After the reaction was completed, it was concentrated by rotary evaporation, and the residue was subjected to column chromatography to obtain 3.23 g of a slightly yellow oily substance with a yield of 83.0%. R f =0.27(Petroleum ether:acetone=20:1).IR(NaCl)3105, 2951, 2805, 1748, 1435, 1200, 1174, 715cm -1 .MSm / z: 394[M+1] + ; 1 H NMR(CDCl 3 )δ = 2.00 (s, 3H), 2.03 (s, 3H), 2.20 ~ 2.57 (m, 6H), 2.89 ~ 2.98 (m, 1H), 3.20 (s, 1H), 3.37 (dd, J = 17.5 / 10.5Hz, 1H), 3.73 (s, 3H), 5.12 (d, J = 45.5 Hz, 1H), 6.04 (t, J = 5.3 Hz, 1H), 6.76 (d, J = 3.5 Hz, 1H), 6.84 (d, J=3.5Hz, 1H), 7.05 (d, J=3.5Hz, 1H), ...
Embodiment 4
[0046] Benzyl α-hydroxyphenylacetate (2.15g, 8.88mmol), TBAT (1.91g, 3.55mmol), triethylamine (3.09ml, 2.24g, 22.2mmol) and 43ml of toluene. After stirring for a few minutes, add PBSF (5.90g) , 19.5mmol), the reaction was stirred for 24h, and the reaction was followed by TLC. After the reaction was completed, it was concentrated by rotary evaporation, and the residue was subjected to column chromatography to obtain 1.97 g of a colorless oily substance with a yield of 90.9%.R f =0.14 (petroleum ether:acetone=160:1).IR (NaCl) 3067, 3036, 2961, 1760, 1683, 1266, 1057, 735, 696cm -1 ; MSm / z: ESI + , 267[M+Na] + ; ESI - , 243[m-1] + ; 1 H NMR(CDCl 3 ) δ=5.21 (dd, J=42.5 / 10 Hz, 2H), 5.82 (d, J=39.5 Hz, 1H), 7.17-7.51 (m, 10H).
[0047] The alcohol hydroxy fluoro derivatives prepared by the fluorination reaction using different hydroxy derivatives as raw materials in the present invention are shown in Table 2:
[0048] Table 2: Fluorination results of various alcohols
[0049]
[0050]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com