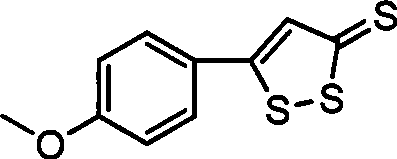
Method for preparing anethol trithione
A kind of technology of anetisol and phosphorus pentasulfide, which is applied in the field of preparation of anetisol, can solve the problems of low yield, difficult to control, high reaction temperature, etc., and achieve the effects of low reaction temperature, few by-products and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Add 48.0ml (0.4mol) of diethyl carbonate and toluene to a 500ml reaction flask, add 33.6g (0.3mol) of potassium tert-butoxide, raise the temperature to 80°C, and slowly add 15.0ml of p-methoxyacetophenone g (0.1mol) toluene solution. After adding, the reaction was completed for 4h. After the reaction was completed, it was lowered to room temperature, poured into water, and the organic layer was separated. The aqueous layer was extracted once with toluene, and the combined organic layers were washed with saturated NaCl solution. The organic layer was a toluene solution containing ethyl p-methoxybenzoyl acetate.
[0031] (2) 26.68g (0.12mol) of phosphorus pentasulfide, 4.8g (0.15mol) of sulfur, 106.5ml (0.5mol) of toluene and hexamethyldisiloxane were added to the reaction flask, and the mixture was heated to reflux. Slowly add the toluene solution of ethyl p-methoxybenzoylacetate prepared above dropwise, and the dropwise reaction is complete. The reaction solution ...
Embodiment 2
[0033] (1) Add 48.0ml (0.4mol) of diethyl carbonate and toluene to a 500ml reaction flask, add 22.4g (0.2mol) of potassium tert-butoxide, raise the temperature to 110°C, and slowly add 15.0ml of p-methoxyacetophenone g (0.1mol) toluene solution. The reaction was completed for 1 h. After the reaction was completed, it was lowered to room temperature, poured into water, and the organic layer was separated. The aqueous layer was extracted once with toluene, and the combined organic layers were washed with saturated NaCl solution. The organic layer was a toluene solution containing ethyl p-methoxybenzoyl acetate.
[0034] (2) Add 22.2 g (0.1 mol) of phosphorus pentasulfide, 4.8 g (0.15 mol) of sulfur, toluene, and 85.3 ml (0.4 mol) of hexamethyldisiloxane into the reaction flask, and heat the mixture to reflux. Slowly add the toluene solution of ethyl p-methoxybenzoylacetate prepared above dropwise, and the dropwise reaction is complete. The reaction solution was cooled, 35.6g o...
Embodiment 3
[0036] (1) Add toluene to the reaction bottle, slowly add 7.2g of sodium hydride (60% content) and 60.0ml (0.5mol) of diethyl carbonate under stirring, raise the temperature to 40 degrees, and slowly add p-methoxyphenyl ethyl Ketone 15.0g (0.1mol) toluene solution. After adding, the reaction was completed for 4h. After the reaction was completed, the reaction solution was poured into ice water, and the pH was adjusted to 9.0 with concentrated HCl. The aqueous layer was extracted with toluene, washed with sodium bicarbonate solution and saturated NaCl solution, and the organic layer was a toluene solution containing ethyl p-methoxybenzoyl acetate.
[0037] (2) Electric stirring 44.4g (0.2mol) of phosphorus pentasulfide, 3.2g (0.1mol) of sulfur, xylene, and 63.3ml (0.3mol) of hexamethyldisiloxane were added to a 500ml reaction flask, and the mixture was heated to reflux. Slowly add the toluene solution of ethyl p-methoxybenzoylacetate (about 0.1 mol) prepared above dropwise, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com