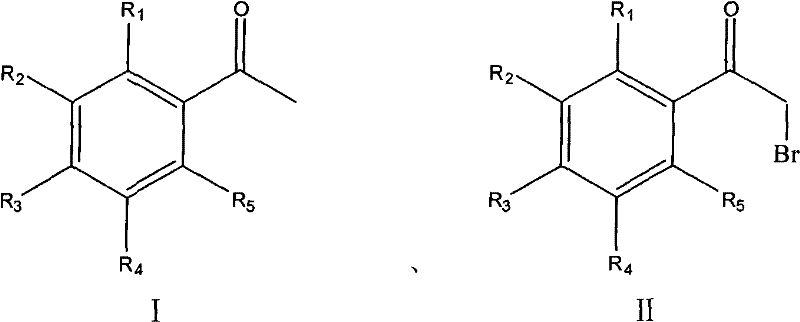
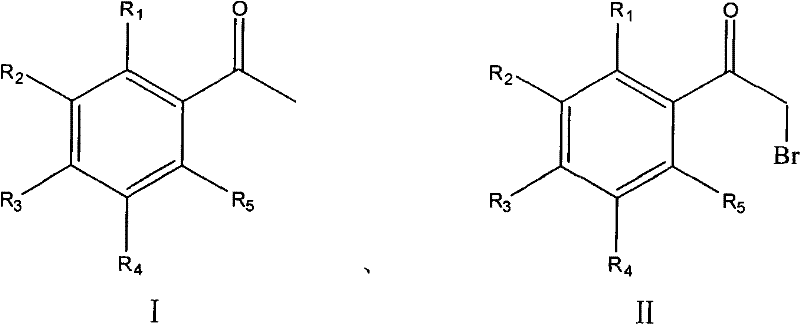
Process for synthesizing alpha-bromoacetophenone compound
A technology of bromoacetophenone and synthesis method, which is applied in the field of synthesis of α-bromoacetophenone compounds, can solve problems such as acid gas corrosion equipment, complex operation, and environmental pollution, and achieve low bromination reagents and high product quality. The effect of high purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Add 36.7 g (220 mmol) of potassium bromate in 100 mL of water and 12.0 g (100 mmol) of acetophenone to a flask, stir, and slowly drop 22.9 g (220 mmol) of 25wt% sodium bisulfite solution into it, about half After adding in hours, control the temperature at 30℃ to react for 6 hours after dripping, cool to room temperature, filter, wash with ice water, recrystallize with n-hexane and dry to obtain white crystals 17.7g, yield 89%, mp48-50℃(lit .49-51°C).
Embodiment 2
[0027] Example 2: Add 36.7g (220mmol) of potassium bromate in 100mL of water and 12.0g (100mmol) of acetophenone to the flask, stir, and slowly drop 21.8g (220mmol) of 10wt% ammonium bisulfite solution into it. After the addition in half an hour, control the temperature at 40°C to react for 6 hours after the dropping, cool to room temperature, filter, wash with ice water, recrystallize and dry with n-hexane to obtain 15.7 g of white crystals, with a yield of 79%.
Embodiment 3
[0028] Example 3: Add 3.0 g (20 mmol) of sodium bromate in 100 mL of water and 1.3 g (9.1 mmol) of p-fluoroacetophenone into the flask, stir, and slowly drop 2.1 g (20 mmol) of 5wt% sulfurous acid into it Add sodium hydrogen solution in about half an hour. After dropping, control the temperature at 50°C to react for 9 hours, cool to room temperature, filter, wash with ice water, recrystallize and dry with cyclohexane to obtain 1.44g of white crystals, with a yield of 73%. mp46-48℃(lit.43-45℃).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com