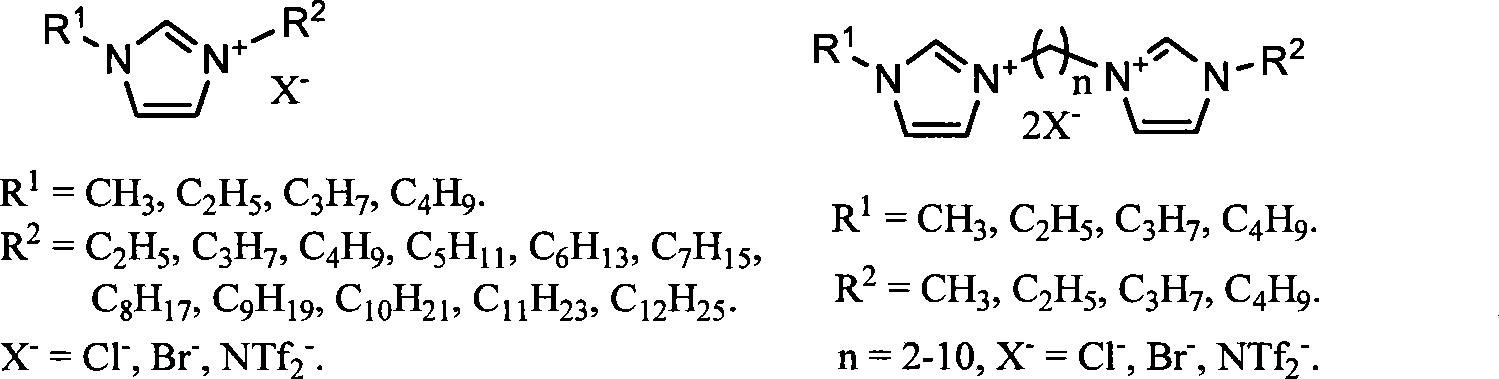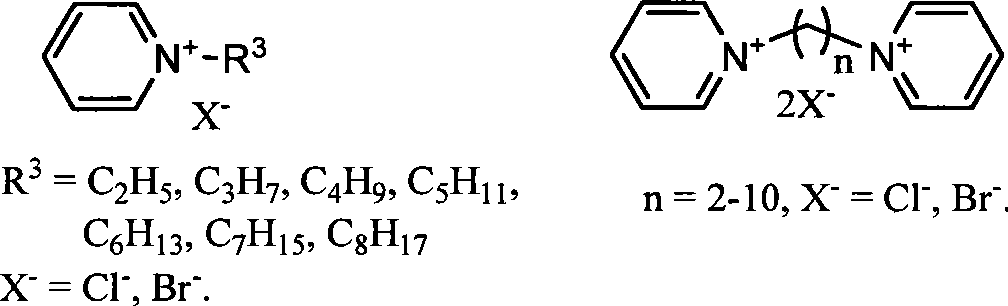Method for preparing 5-hydroxymethyl-furfural
A technology of hydroxymethyl furfural and ionic liquid, applied in the production field of 5-hydroxymethyl furfural, can solve the problem of lack of efficient and low-cost reaction system, effective separation and purification of HMF, unfavorable industrial production of HMF, large amount of silica gel, etc. problems, to achieve the effect of less catalyst and solvent consumption, less requirements for corrosion resistance, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
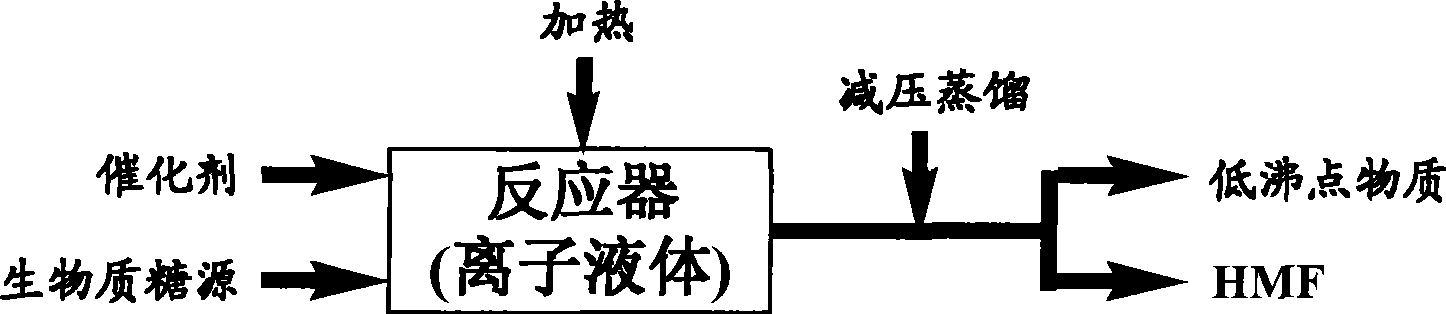
Image
Examples
Embodiment 1
[0040] Add 20 grams of ionic liquid [C4MIm]Cl into the reactor, heat to 100°C, add 10 grams of fructose, and then add 0.25 grams of concentrated sulfuric acid, and continue the reaction at 100°C for 20 minutes under normal pressure before stopping.
[0041] The reaction system was directly decompressed to 133Pa, and the 114-116°C fraction was collected by distillation to obtain 6.6 grams of a dark yellow liquid product. The nuclear magnetic resonance (NMR) analysis data of this product were: 1 H NMR (400MHz, CDCl 3 ): δ 9.49(s, 1H), 7.20(d, 1H), 6.48(d, 1H), 4.65(s, 2H); 13 CNMR (100MHz, CDCl 3 ): δ 178.2, 161.6, 152.5, 123.9, 110.4, 57.7. The measured data of the product is completely consistent with the NMR data obtained by using the HMF reagent provided by Sigma, and then analyzed by ultraviolet-visible spectroscopy, it is found that the absorption peak is 282nm, which is consistent with the literature report, confirming that the product is HMF, and the yield is 94%.
[...
Embodiment 2
[0044] 20 g of ionic liquid [C 4 MIm]Cl was added to the reactor, heated to 80°C, 10 grams of fructose was added, followed by 0.25 grams of concentrated sulfuric acid, and the reaction was stopped after 30 minutes at 100°C.
[0045] The reaction system was directly distilled under the operating pressure of 2.0KPa, and the low-boiling substances were distilled off at 80°C, and the pressure was continued to 3.0Pa, and the fraction at 110°C was collected to obtain 6.5 grams of dark yellow liquid product. The nuclear magnetic resonance spectrum (NMR) analysis data of the product The analysis data of ultraviolet-visible spectrum is the same as that of Example 1, and it is confirmed that the product is HMF, and the yield is 93%.
[0046] The vacuum distillation residue was returned to normal pressure and cooled to 80° C., 10 g of fructose and 0.25 g of concentrated sulfuric acid were added, and the reaction and product separation were carried out as described above to obtain 6.3 g o...
Embodiment 3
[0048] 20 g of ionic liquid [C 4 MIm]Cl was added to the reactor, heated to 100°C, 20 g of fructose was added, followed by 0.5 g of concentrated sulfuric acid, and the reaction was continued at 100°C for 30 minutes before stopping. The reaction system was decompressed to 2.0KPa, and low-boiling substances were distilled off at 80°C; the pressure was continued to 267Pa, and fractions at 141°C to 142°C were collected by distillation to obtain 12.5 grams of a dark yellow liquid product, which was analyzed by nuclear magnetic resonance (NMR) The analysis data of ultraviolet-visible spectrum is the same as that in Example 1, and it is confirmed that the product is HMF, and the yield is 89%.
[0049] The distillation residue was returned to normal pressure and cooled to 100° C., 20 g of fructose and 0.5 g of concentrated sulfuric acid were added, and reacted and separated as described above to obtain 11.8 g of a dark yellow liquid product with a HMF yield of 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com