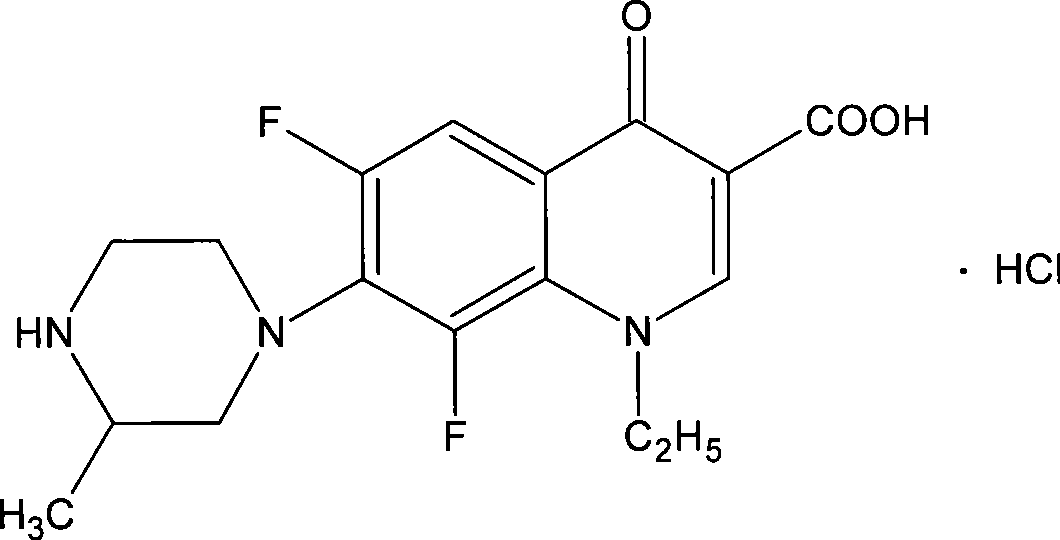
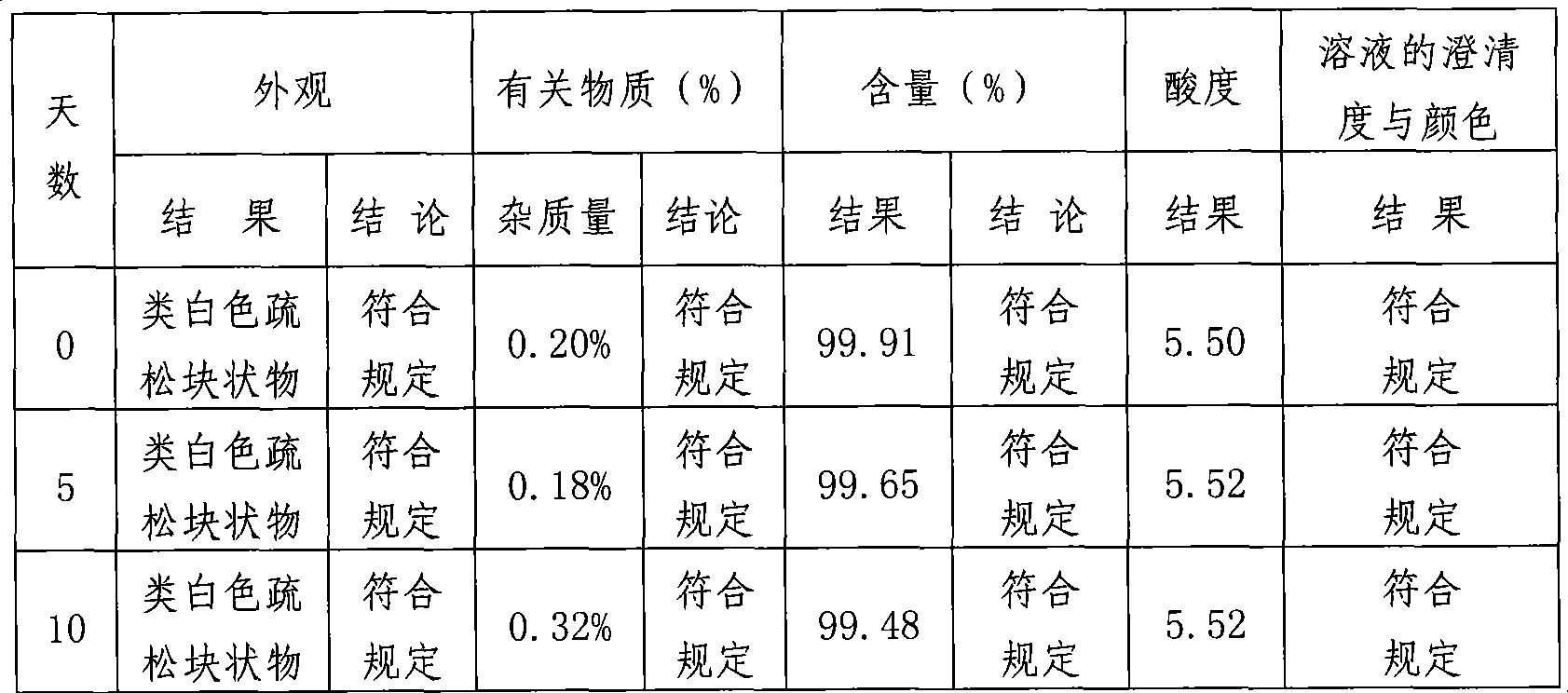
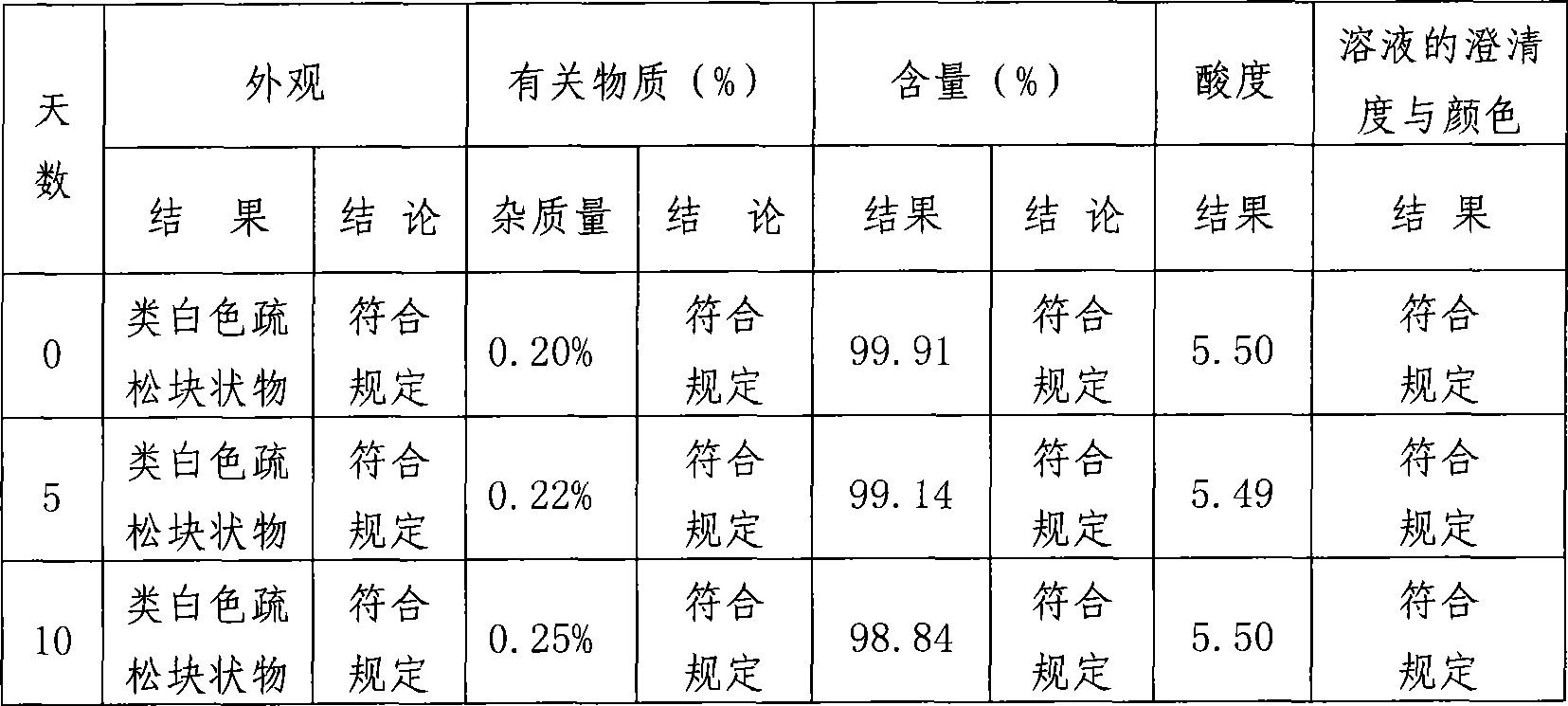
Lomefloxacin hydrochloride lyophilized powder for injection and preparation method therefor
A technology of lomefloxacin hydrochloride and freeze-dried powder injection, which is applied in the field of lomefloxacin hydrochloride freeze-dried powder injection for injection and its preparation, can solve the difference of product formability and resolubility, affect product quality and curative effect, and pH value fluctuation It can achieve the effect of good tolerance, low incidence of adverse reactions, and low toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of 0.1g Lomefloxacin Hydrochloride Injection Freeze-dried Powder Injection
[0049] Prescription composition: (1000 bottles of prescription volume)
[0050] Lomefloxacin hydrochloride (pure product is calculated as lomefloxacin) 100g
[0051] Mannitol 41.4g
[0052] Glacial acetic acid-sodium acetate buffer (pH 4.5) 2600ml
[0053] Filled in 7ml vials
[0054] Preparation of glacial acetic acid-sodium acetate buffer (pH 4.5): Take 9.8ml of glacial acetic acid, add 18g of sodium acetate, and dilute to 1000ml with water for injection.
[0055] Add the prescribed amount of buffer into the concentrated tank and heat to about 70°C.
[0056] Add the prescribed amount of mannitol into the concentrated tank and stir to dissolve.
[0057] Slowly and evenly add the prescribed amount of lomefloxacin hydrochloride into the concentrated tank and stir to dissolve, then cool to about 60°C.
[0058] Measure the pH value of the liquid, and adjust the pH value...
Embodiment 2
[0068] Example 2 Preparation of 0.2g Lomefloxacin Hydrochloride Injection Freeze-dried Powder Injection
[0069] Prescription composition: (1000 bottles of prescription volume)
[0070] Lomefloxacin hydrochloride (pure product is calculated as lomefloxacin) 200g
[0071] Mannitol 82.8g
[0072] Glacial acetic acid-sodium acetate buffer (pH 4.5) 5400ml
[0073] Potting in 12ml vials
[0074] Preparation of glacial acetic acid-sodium acetate buffer (pH 4.5): Take 9.8ml of glacial acetic acid, add 18g of sodium acetate, and dilute to 1000ml with water for injection.
[0075] Add the prescribed amount of buffer into the concentrated tank and heat to about 70°C.
[0076] Add the prescribed amount of mannitol into the concentrated tank and stir to dissolve.
[0077] Slowly and evenly add the prescribed amount of lomefloxacin hydrochloride into the concentrated tank and stir to dissolve, then cool to about 60°C.
[0078] Measure the pH value of the liquid, and adjust the pH val...
Embodiment 3
[0088] Example 3 Preparation of 0.4g lomefloxacin hydrochloride freeze-dried powder for injection
[0089] Prescription composition: (1000 bottles of prescription volume)
[0090]Lomefloxacin hydrochloride (pure product is calculated as lomefloxacin) 400g
[0091] Mannitol 165.6g
[0092] Glacial acetic acid-sodium acetate buffer (pH 4.5) 10400ml
[0093] Filled in 30ml vials
[0094] Preparation of glacial acetic acid-sodium acetate buffer (pH 4.5): Take 9.8ml of glacial acetic acid, add 18g of sodium acetate, and dilute to 1000ml with water for injection.
[0095] Add the prescribed amount of buffer into the concentrated tank and heat to about 70°C.
[0096] Add the prescribed amount of mannitol into the concentrated tank and stir to dissolve.
[0097] Slowly and evenly add the prescribed amount of lomefloxacin hydrochloride into the concentrated tank and stir to dissolve, then cool to about 60°C.
[0098] Measure the pH value of the medicinal solution, adjust the pH v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com