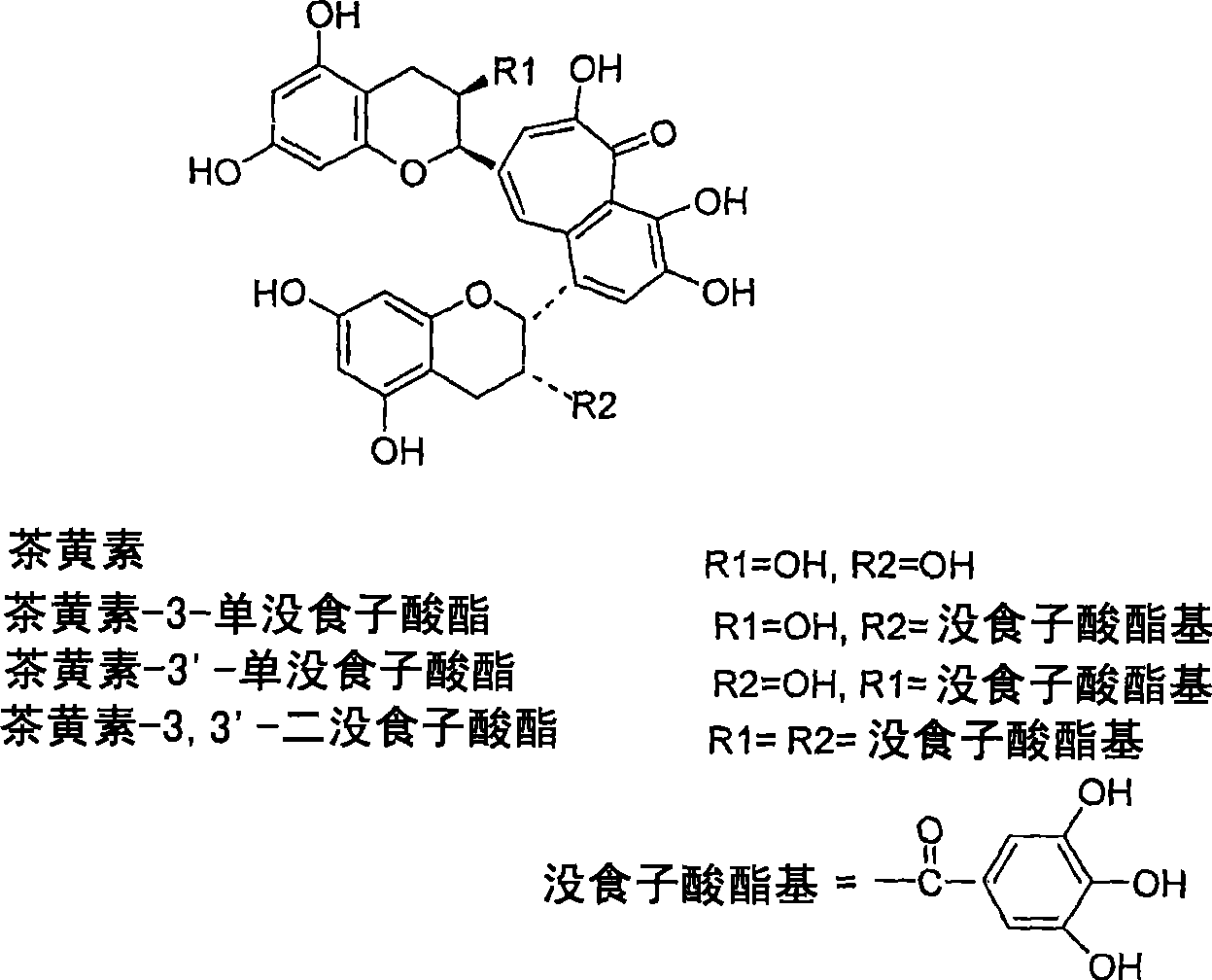
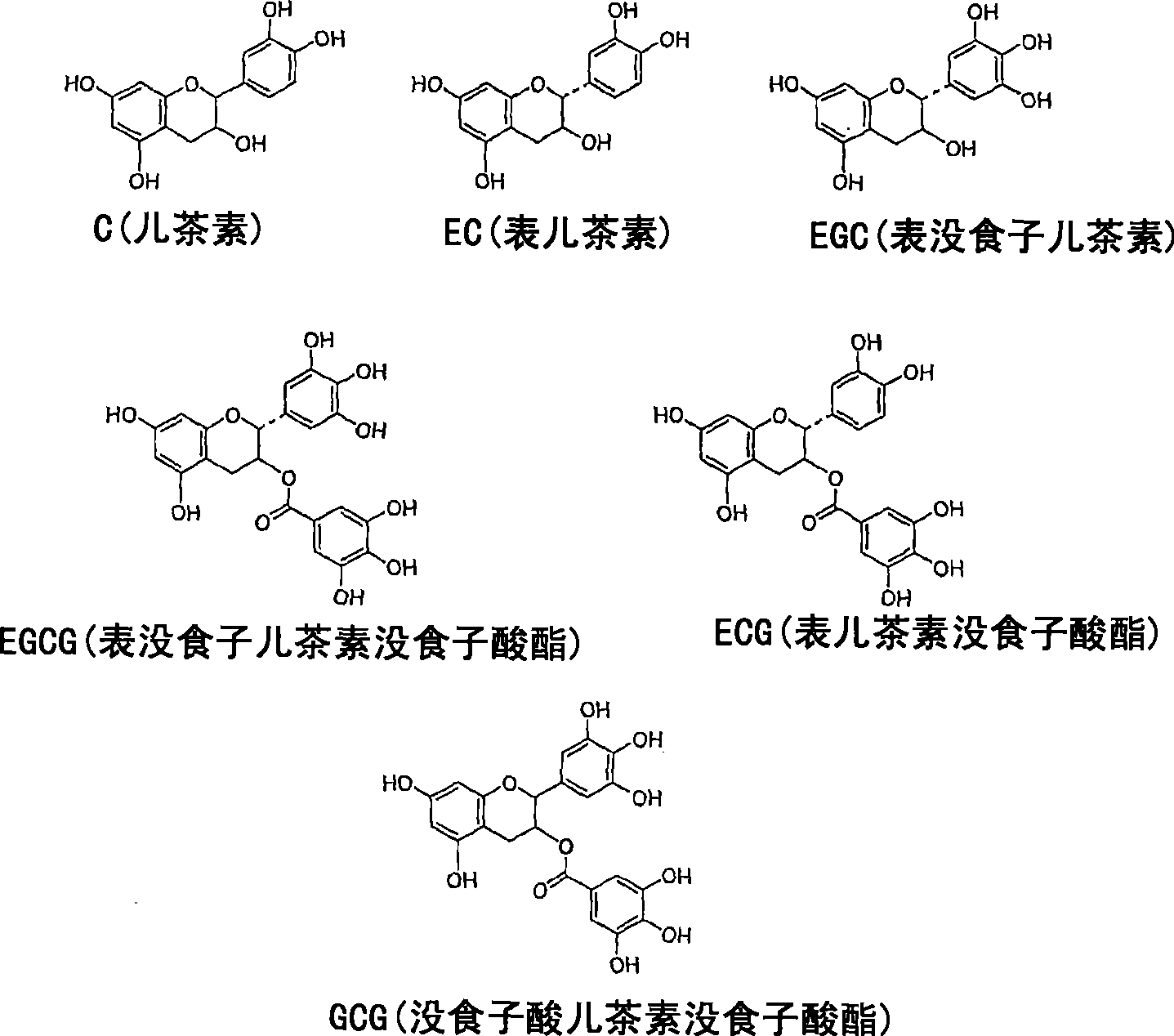
A solid state matrix, process of preparation thereof, and process of preparation of theaflavins
一种茶黄素、基材的技术,应用在生物化学设备和方法、固定在有机载体上/在其中的、酵素等方向,能够解决靶分子失活、丧失生物学活性、不适合用作生物反应器或反复使用的材料等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0088] 1. The method as described above, comprising, in step (c), reacting the polymer activated by carbonyldiimidazole with a target moiety at a predetermined molar ratio and at a predetermined molar concentration, thereby producing a mixture of the polymer and the target moiety :1 polymer:target adduct, and recover the adduct according to step (d).
[0089] 2. A method as described above, comprising, in step (c), reacting an activated polymer with a target moiety having a plurality of reactive groups at a predetermined molar ratio and molar concentration, thereby producing such a polymer and target Adducts of Adducts: In which a preselected proportion of reactive groups have reacted and attached to a polymer molecule, especially for macromolecular targets. The preselected proportion may be, for example, 1-100%, preferably 10-90%, such as 10, 20, 30, 40, 50, 60, 70, 80 or 90%.
[0090] 3. The method as described above, comprising, in step (c), reacting the polymer activated ...
Embodiment I
[0130] In a plastic beaker, 0.3 g of substrate (CDI-activated acrylic (ester) polymer resin) was resuspended in 3 ml of pH 6.2 phosphate buffer, and 2.0 ml of crude polyphenol oxidase (1.66 mg ×2-enzyme protein); at 25°C, incubate the whole mixed system in a shaking incubator at 100r.p.m. for 1 hour; after incubation, filter the mixture through glass wool, and detect the enzyme in the filtrate with tea source substrate Activity, it was found that there was no enzyme activity at all, indicating that all enzyme proteins were bound to the substrate; then the immobilized enzyme bound to the substrate was washed with 10ml of water, and the remaining enzyme activity in the filtrate was detected again, no enzyme activity was detected in the filtrate, indicating that the enzyme Covalently bond with the substrate; add 0.04g tea source substrate dissolved in 4.0ml water to the above substrate in a plastic beaker, then incubate in a shaking incubator at 37.5°C, 100r.p.m. for half an hour,...
Embodiment -II
[0135] In a plastic beaker, 0.5 g of the base material (CDI-activated acrylate polymer resin) was suspended in 5 ml of phosphate buffer at pH 6.2, and 5.0 ml of crude lychee (litchi chinensis) bud extracted Polyphenol oxidase (1.15 mg × 2-enzyme protein); 20 ° C, the whole mixed system was incubated in a shaking incubator at 100 r.p.m. for 2 hours; The enzyme activity in the filtrate was detected by the substrate, and it was found that there was no enzyme activity at all, indicating that all enzyme proteins were combined with the substrate; then the immobilized enzyme bound to the substrate was washed with 10ml of water, and the residual enzyme activity in the filtrate was detected again, and in the filtrate No detection of enzyme activity indicates that the enzyme is covalently bound to the substrate; add 0.05 g of lychee substrate dissolved in 5.0 ml of water to the above substrate in a plastic beaker, and then incubate in a shaking incubator at 25 ° C and 100 r.p.m. After i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com