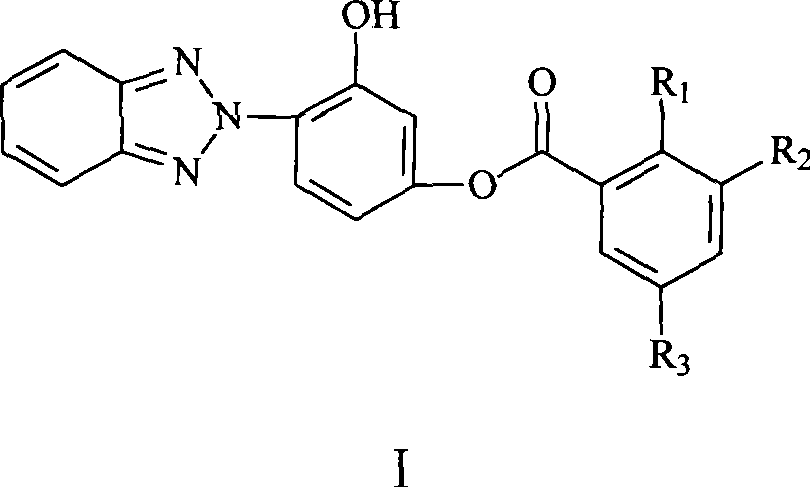
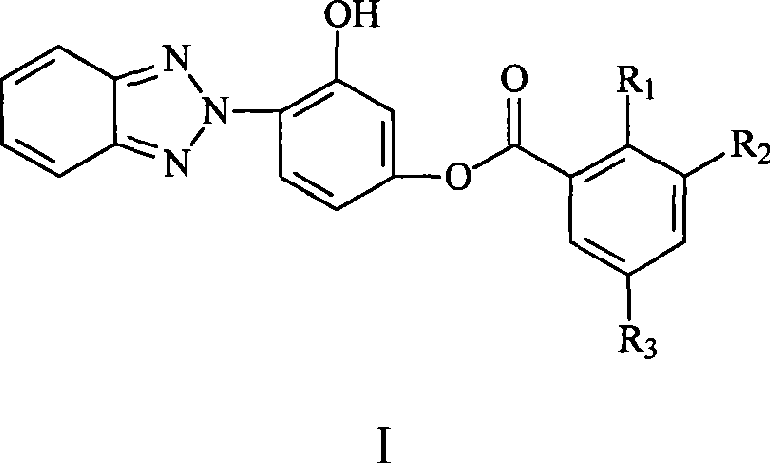
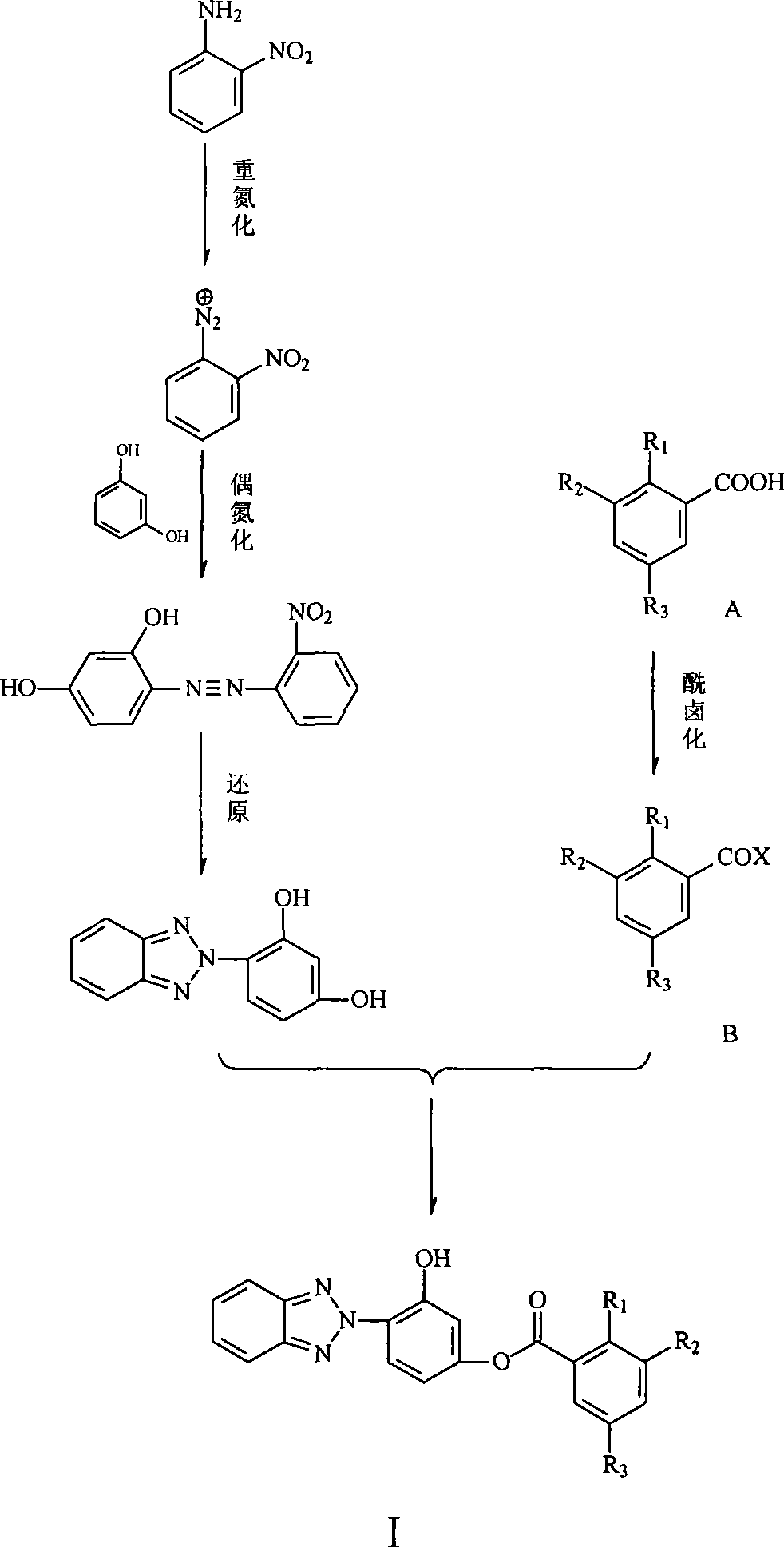
Phenyl (benzotriazolyl) benzoate compound
A technology of benzotriazole base and compound, which is applied in the field of phenyl benzoate compounds to achieve the effects of not easy migration and loss, and excellent ultraviolet absorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Synthesis of 2-hydroxybenzoic acid-(3'-hydroxy-4'-benzotriazolyl)phenyl ester
[0018] (1) Preparation of o-nitroaniline diazonium salt:
[0019] In a 250ml three-necked flask with a thermometer and a stirring rod, add 4.14g of o-nitroaniline, 10.3ml of concentrated HCl and 5ml of water, stir rapidly, and cool the reaction mixture to 0°C to 5°C under ice-salt bath conditions, add 2.1g NaNO 2 Make an aqueous solution with 4ml of water, keep it at 0℃~5℃ for 30 minutes, add a small amount of urea to remove excess nitrous acid. Stir until starch potassium iodide shows a light blue color, filter to remove insoluble matter, and place the diazonium salt solution in an ice-salt bath for later use.
[0020] (2) Preparation of 2-nitro-2', 4'-dihydroxyazobenzene:
[0021] In a 250ml three-neck flask equipped with a thermometer and a stirring rod, add 3.63g (0.033mol) of resorcinol and 40ml of water. Use an ice-salt bath to control the temperature at 0°C to 5°C, and add the o-n...
Embodiment 2
[0032] Synthesis of 2-hydroxy-3,5-dibromobenzoic acid-(3'-hydroxy-4'-benzotriazolyl)phenyl ester
[0033] (1) 3,5-Dibromosalicylic acid
[0034] Add 13.8g of salicylic acid and 100ml of glacial acetic acid into a 250ml three-necked flask equipped with a stirring rod, and slowly add dropwise a solution made of 12.8ml of liquid bromine and 50ml of glacial acetic acid. After the addition is complete, control the reaction temperature below 25°C. The reaction was continued for 1 hour. 80 ml of ice water was added to the reaction mixture with stirring, and the reaction mixture was cooled to 10°C-15°C. Stand still and think about it. Then washed with dilute acetic acid and water, and dried to obtain 28 g of white solid 3,5-dibromosalicylic acid, yield 95%, m.p 218°C-220°C.
[0035] (2) 3,5-dibromosalicyloyl chloride
[0036] Add 2.96 grams of 3,5-dibromosalicylic acid into a 50ml three-necked flask equipped with a reflux condenser, add 20ml of ethylene glycol dimethyl ether, and ...
Embodiment 3
[0043] Synthesis of 2-hydroxy-3,5-dinitrobenzoic acid-(3'hydroxy-4'-benzotriazolyl)phenyl ester
[0044] (1) 3, the preparation of 5-dinitrosalicyloyl chloride:
[0045] Add 2.28 grams of 3,5-dinitrosalicylic acid, 20 ml of ethylene glycol dimethyl ether, and catalytic amount of pyridine in a 50 ml three-necked flask equipped with a reflux condenser, connect the exhaust gas absorption tube above the condenser tube, and put the tail gas absorption tube in the exhaust gas absorption bottle. Filled with 10% NaOH aqueous solution. Heat up to 60°C under stirring, add dropwise a solution made of 1.5ml thionyl chloride and 5ml ethylene glycol dimethyl ether with a constant pressure dropping funnel, control the rate of addition, finish dropping in about 30 minutes, maintain the temperature and continue React for 1 hour. The temperature was raised to reflux, and the reaction was continued for 6 hours. Stop the reaction, distill off the solvent and a small amount of unreacted thionyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com