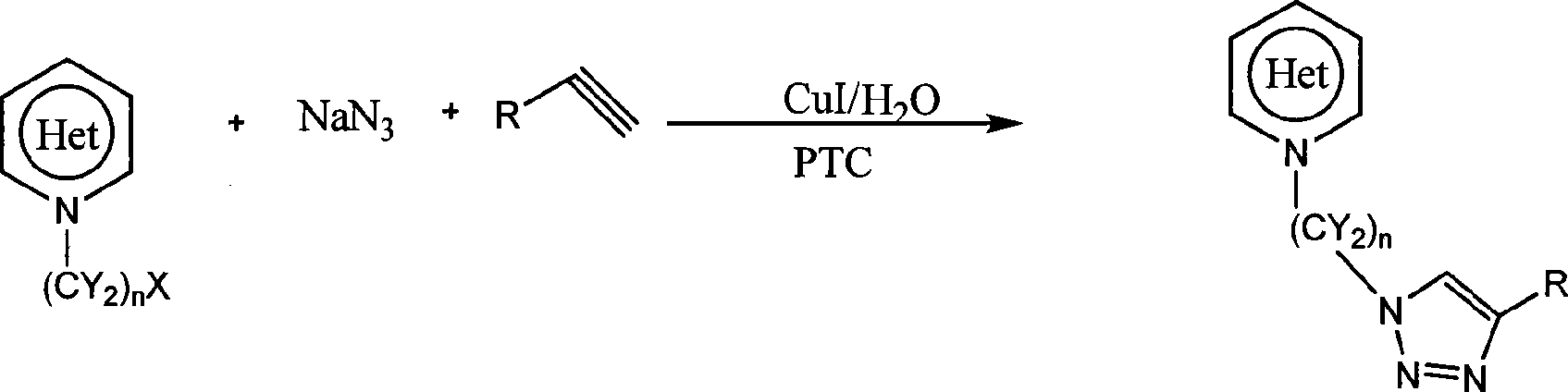
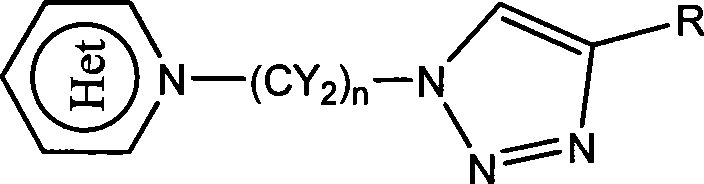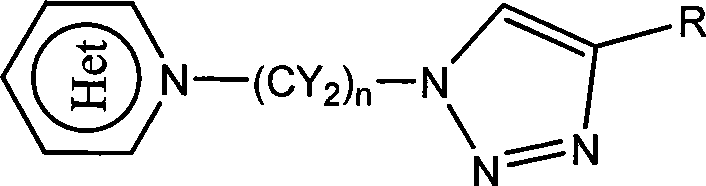Heterocycle substituted triazole compound and synthetic method thereof
A compound, triazole technology, applied in the field of pharmaceutical intermediate compounds and their synthesis, can solve the problems of long reaction time, large environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. The preparation of 1-(2-chloroethyl)indole, according to the literature Dariusz Bogdal and Knysztof Jaskot, SYNTHETIC COMMUNICATIONS, 2000, 30(18), 3341-3352.
[0031] 2. Suspend 1-(2-chloroethyl)indole (0.2mol) in 20ml of water, add sodium azide (0.24mol), phenylacetylene (0.22mol) and cuprous iodide (0.006mol), and finally Add PEG-600 (0.04mol), stir at 20-30°C for 8 hours, filter, wash with dilute ammonia (2 x 15ml), wash with water (2 x 10ml), wash with hexane (3 x 10ml) and dry. Yield 79%. 1 H NMR (500MHz, CDCl 3 )δ 7.63-7.65(d, 2H, J=7.8Hz), 7.59-7.61(m, 3H), 7.11-7.37(m, 4H), 6.93(s, 1H), 6.75(d, 1H, J=2.65 Hz), 6.45(d, 1H, J=2.35Hz), 4.74(t, 2H, J=10.85Hz), 4.65(t, 2H, J=10.95Hz); 13 C NMR (500MHz, CDCl 3 )δ 46.91, 50.29, 102.91, 109.10, 120.49, 121.22, 121.82, 122.63, 126.15, 128.60, 129.17, 130.70, 135.88, 148.30;
Embodiment 2
[0033] 1. Preparation of 2-bromomethylbenzimidazole. In the there-necked flask of 100 milliliters that is added with 60 milliliters of carbon tetrachlorides, add 2-methylbenzimidazole (0.42mol), NBS (0.45mol) and benzoyl peroxide (1mol%), under magnetic stirring (Nitrogen was introduced as a protective gas), heated to reflux for 5 hours until the reaction was complete (TLC detection), and the product was obtained with a yield of 97%.
[0034] 2. Suspend 2-bromomethylbenzimidazole (0.2mol) in 20ml of water, add sodium azide (0.24mol), phenylacetylene (0.22mol) and cuprous iodide (0.005mol), and finally add TBAB ( 0.05mol), stirred at 20-30°C for 3 hours, filtered, washed with dilute ammonia (2 x 15ml), washed with water (2 x 10ml), washed with hexane (3 x 10ml) and dried. Yield 93%. m.p.210~211℃. MS m / z=275; 1 H NMR (500MHz, DMSO) δ12.70(s, 1H), 8.71(s, 1H), 7.90(d, 2H, J=7.55Hz), 7.62(d, 2H), 7.44-7.52(m, 2H) , 7.21-7.36(m, 3H), 5.94(s, 2H); 13 CNMR (500MHz, DMSO) δ 47.96...
Embodiment 3
[0036] 1. Preparation of 9-(2-chloroethyl)carbazole. According to Dariusz Bogdal and Knysztof Jaskot, SYNTHETIC COMMUNICATIONS, 2000, 30(18), 3341-3352.
[0037] 2. Suspend 9-(2-chloroethyl)carbazole (0.2mol) in 20ml of water, add sodium azide (0.24mol), phenylacetylene (0.22mol) and cuprous iodide (0.005mol), and finally Add TBAB (0.05mol), stir and react at 20-30°C for 10 hours, filter, wash with dilute ammonia (2 x 15ml), water (2 x 10ml), hexane (3 x 10ml) and dry. Yield 82%. MS m / z = 338. 1 H NMR (500MHz, CDCl 3 )δ 7.99(d, 2H, J=7.7Hz), 7.92(d, 2H, J=7.75Hz), 7.40-7.58(m, 5H), 7.00-7.38(m, 4H), 6.91(d, 2H) , 4.92(t, 2H, J=11.7Hz), 4.87(t, 2H, J=11.45Hz);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com