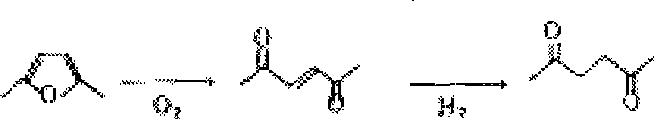Method for synthesizing 2,5-acetonyl acetone
A synthesis method, the technology of hexanedione, which is applied in the field of organic compound synthesis, can solve problems such as environmental pollution, and achieve the effects of cost reduction, purity improvement, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A method for synthesizing 2,5-hexanedione, the reaction scheme is
[0051]
[0052] Follow the steps in the following order:
[0053] a. Preparation of 2,5-hexanedione
[0054] Add 1000g of raw material 2,5-dimethylfuran into the four-neck flask, add 400g of glacial acetic acid, 480g of water, and 30mL of 10% sulfuric acid, reflux at 82°C for 48 hours, take a sample and extract it with ethyl acetate, and detect it by gas chromatography. The raw material content is 1.5% (less than 2%), and the temperature is lowered to obtain the reaction solution A; wherein the gas chromatography detection condition is:
[0055] Chromatographic column: SE-30 capillary column, 30m×0.25mm×0.25μm
[0056] Column temperature: 110°C / 5min—>20°C / min—>260°C / 5min;
[0057] Vaporization chamber temperature: 260°C; detection chamber temperature: 260°C;
[0058] Carrier gas pressure (N 2 ): 80kpa; Injection volume: 0.2μL;
[0059] Quantitative method: peak area normalization method; split ...
Embodiment 2
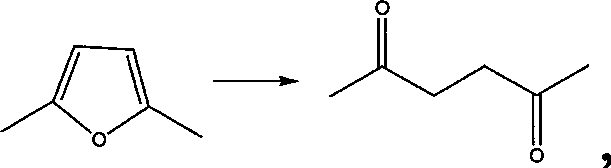
[0070] A method for synthesizing 2,5-hexanedione, the reaction scheme is
[0071]
[0072] Follow the steps in the following order:
[0073] a. Preparation of 2,5-hexanedione
[0074] Add the 10% sulfuric acid of raw material 2,5-dimethylfuran 990g, glacial acetic acid 170g, water 120g, 30mL in the four-necked bottle, and add the gained F10g of embodiment 1, G411g (containing 82g acetic acid, 328g water) and 180g D ( Containing 148g acetic acid, 32g water), refluxed at 82 ℃ for 48 hours, extracted with ethyl acetate after sampling, then utilized the same gas chromatography as Example 1 to detect the raw material content, the measured result was 1.7% (less than 2%), The temperature was lowered to obtain the reaction solution A.
[0075] b. Removal of the previous fraction
[0076] Add 9 g of sodium acetate to the reaction solution A, and stir at room temperature for 30 minutes. Atmospheric distillation to remove 410g of fraction B before 110°C, then add 120g of water to ...
Embodiment 3-6
[0083] The difference between Embodiment 3-6 and Embodiment 1 lies in the difference in the amount of substances used and the different reaction conditions, which can refer to the above table. Of course, corresponding data such as the amount of reaction product, the raw material content measured before cooling in step a, chromatogram, product yield and purity, acetic acid content in G and D must be different because of the above data differences (not shown in the table) out).
[0084] In their synthetic method steps, the remaining contents are consistent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Injection volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com