High efficiency recombinant expressed chitoanase
A chitosanase and amino acid technology, applied in the field of chitosanase, molecular biology and genetic engineering, can solve the problems of increased difficulty, large burden of expression host, low expression level, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
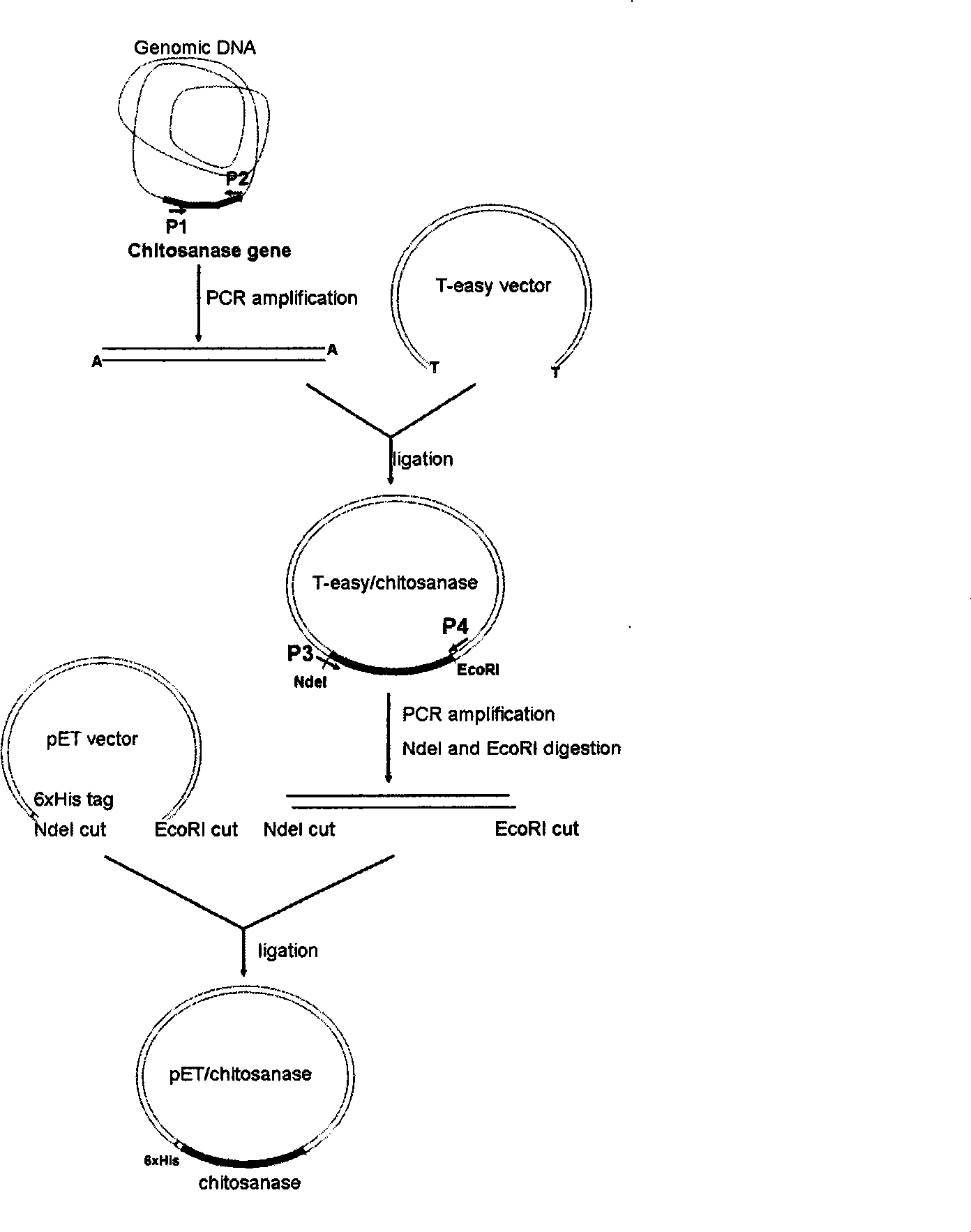
[0025] Construction of chitosanase expression vector
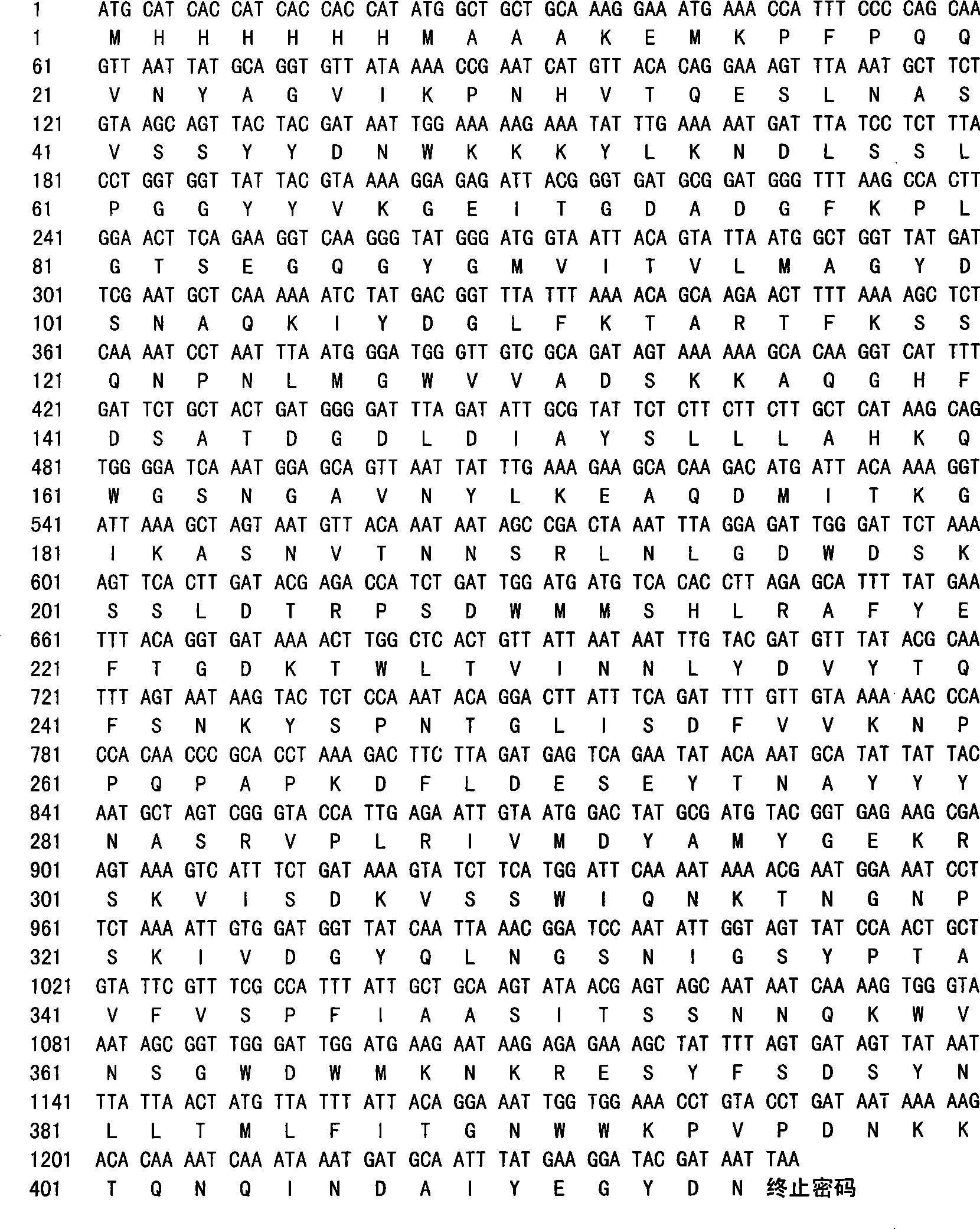
[0026] Genomic DNA was extracted from Gram-positive bacillus strain Bacillus A520 (purchased from Zhejiang Fengan Biopharmaceutical Co., Ltd.), and the genomic DNA was used as a template, and oligonucleotides P1 and P2 were used as primers to amplify the coding The DNA fragment of the truncated chitosanase.
[0027] Specifically, the nucleotide sequence of the primer P1 used is: 5'-GCT GCT GCA AAG GAA ATGAAA-3'; the nucleotide sequence of the primer P2 used is: 5'-TTAATT ATC GTA TCC TTC ATA AATT-3' . The PCR cycle parameters are: 94°C, 30 seconds; 50°C, 50 seconds; 72°C, 120 seconds, a total of 30 cycles. The final step was an incubation at 72°C for 15 minutes. The amplified product was identified by agarose gel, and connected with T carrier (purchased from Promega). The ligation product was transformed into Escherichia coli DH12S strain (available from Invitrogen). Pick several transformed clones to extract plasmids,...
Embodiment 2
[0029] Induced Expression of Recombinant Chitosanase
[0030] The pET / chitosanase plasmid constructed in Example 1 was used to transform Escherichia coli expression strain BL21(DE3) (available from Invitrogen), and the transformed clone was grown on solid LB medium containing ampicillin (100 μg / ml). A single clone was picked and inoculated in 50 ml liquid LB medium (containing 100 μg / ml ampicillin), and cultured overnight at 37° C. with shaking (rotating speed 250 rpm). Inoculate 15ml of the above bacterial solution into 500ml of liquid LB medium (containing 100μg / ml of ampicillin), culture at 37°C with shaking (250rpm) until the absorbance of the bacterial solution at 600nm is 0.6-1.0, and then add IPTG to the final The concentration is 1 mmol / l. The bacterial solution was incubated at 37° C. with shaking (160 rpm) for about 18 hours. For high-density shake flask culture, culture Escherichia coli in 500ml liquid LB medium (containing 100μg / ml ampicillin) at 37°C with shakin...
Embodiment 3
[0032] Purification of recombinant chitosanase
[0033] 500 milliliters of the bacterium solution centrifuged (5000g, 10min) of embodiment 2 high-density induced expression, remove supernatant, bacterium uses 50 milliliters of lysis buffers (20mmol / l sodium phosphate solution containing 0.5mol / l, pH7.5) Wash the cells. Centrifuge again (5000g, 10min), and the cells are resuspended with 30ml of the above-mentioned lysis buffer. The resuspended bacteria were disrupted on ice by ultrasonic (power 200 watts, ultrasonic for 5 seconds, with an interval of 2 seconds, and the total ultrasonic time was 20 minutes). The broken bacteria liquid was centrifuged (8000g, 10min), and the supernatant was taken, which contained recombinant chitosanase. The supernatant was split 3 times onto metal chelate (Ni 2+ ) chromatography column (available from Pharmacia) (diameter 1.5 cm, column height 6 cm), flow rate 1 ml per minute. Then rinse with a lysis buffer containing 20mmol / l imidazole, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com