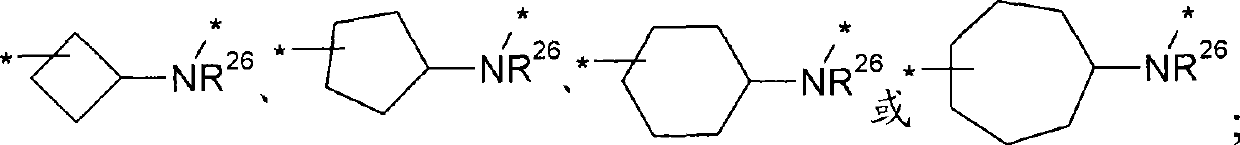
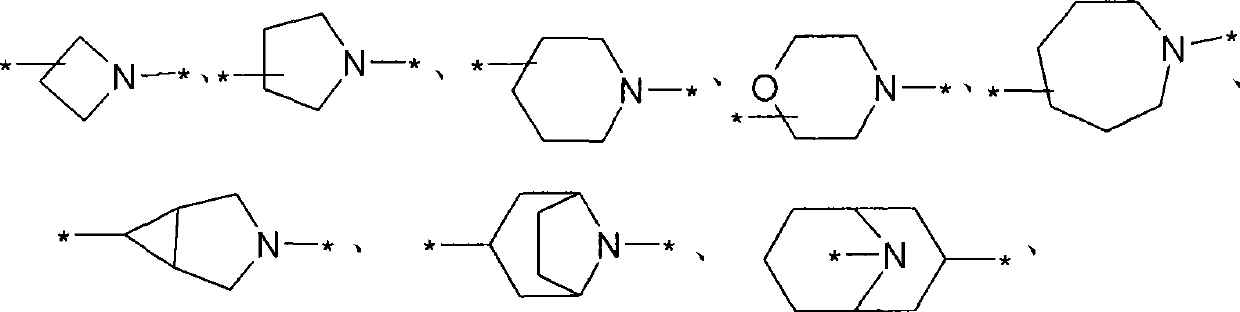
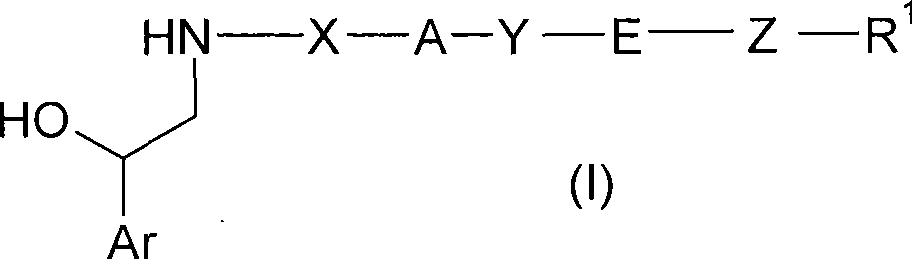Phenethanolamine derivatives as beta2 adrenoreceptor agonists
A halogen and compound technology, applied in drug combinations, active ingredients of heterocyclic compounds, respiratory diseases, etc., can solve problems such as slow onset of effect, hindering rescue treatment and maintenance treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0510] 8-Hydroxy-5-{(1R)-1-hydroxyl-2-[({1-[2-(2-phenylethoxy)ethyl]piperidin-4-yl}methyl)amino]ethyl Base} quinolin-2(1H)-one
[0511]
[0512] i) [1-[2-(2-phenylethoxy)ethyl]-piperidin-4-yl]-methanol
[0513] A solution of 2-phenylethoxyacetaldehyde (WO 94 / 27601) (0.72 g) and piperidine-4-methanol (0.5 g) in methanol (20 mL) was treated with AcOH (20 mg) and stirred at room temperature for 30 minutes. At the end, sodium cyanoborohydride (103 mg) was added and the mixture was stirred at room temperature for 18 hours. The reaction mixture was added by adding concentrated NH 3 The aqueous solution (1 mL) was basified and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (using 1% concentrated NH 3 aqueous and 5% ethanol in DCM) to afford the subtitle compound. Yield: 0.1 g.
[0514] 1 H NMR (CDCl 3 )δ 7.30-7.18 (m, 5H), 3.67-3.64 (m, 2H), 3.60-3.57 (m, 2H), 3.49-3.47 (m, 2H), 2.95-2.87 (m, 4H), 2.58-2.55 (m, 2H),...
Embodiment 2
[0523] 8-Hydroxy-5-{(1R)-1-hydroxy-2-[(trans-4-{[2-(2-phenylethoxy)ethyl]amino}cyclohexyl)amino]ethyl} Quinolin-2(1H)-one
[0524]
[0525] i) 8-(Benzyloxy)-5-(bromoacetyl)quinolin-2(1H)-one
[0526]To a solution of 5-acetyl-8-(benzyloxy)quinolin-2(1H)-one (WO2005 / 123684) (18.05 g) in DCM (200 mL) was added dropwise trifluorotrifluoroethylene over 15 minutes at 0°C Boronide etherate (9.2 mL), the mixture was then warmed to room temperature to form a thick yellow suspension. The mixture was heated at 40 °C and a solution of bromine (3.4 mL) in DCM (100 mL) was added slowly over 40 min. After an additional 15 minutes, the mixture was allowed to come to room temperature and the volatiles were removed on a rotary evaporator. The residue was triturated with excess 10% aqueous sodium carbonate for 1 hour. The gummy solid was collected by filtration and further washed with H 2 O washed, and the solid was dried under vacuum at 40 °C overnight. The solid was purified by furthe...
Embodiment 3
[0549] 8-Hydroxy-5-[(1R)-1-hydroxy-2-({1-[3-(2-phenylethoxy)propyl]piperidin-4-yl}amino)ethyl]quinoline -2(1H)-one
[0550]
[0551] i) 4-[(1R)-2-azido-1-hydroxyethyl]-8-(benzyloxy)quinolin-2(1H)-one
[0552] Sodium iodide (0.47 g) and sodium azide (0.74 g) were added to the product (8-(benzyloxy)-5-[(1R)-2-bromo-1- Hydroxyethyl] quinolin-2(1H)-one) (1.07g) in anhydrous DMSO (10mL). The reaction mixture was heated at 65 °C for 2 h. The mixture was allowed to cool to room temperature, then washed with EtOAc and H 2 O was diluted and the layers were separated. The aqueous material was further extracted with EtOAc (x4), and the combined organic extracts were then washed with saturated aqueous NaCl. The organic phase was collected and dried (Na 2 SO 4 ) followed by removal of volatiles in vacuo to yield a yellow solid. The solid residue was purified by trituration with 1:1 ether / EtOAc to afford the subtitle compound as a white solid. Yield: 0.71 g.
[0553] MS APCI+33...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com