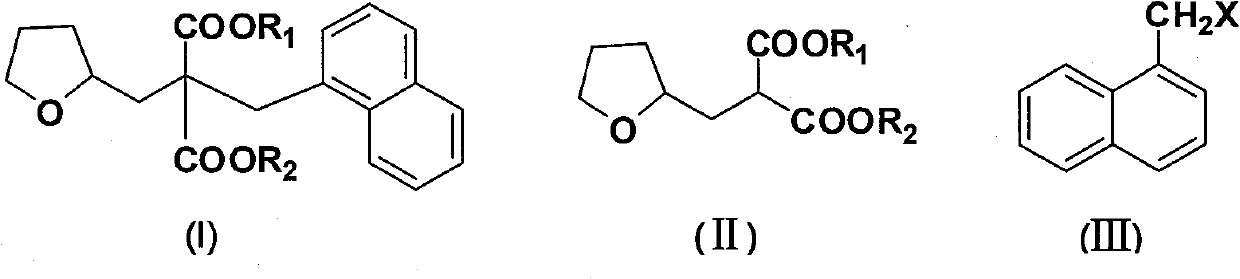
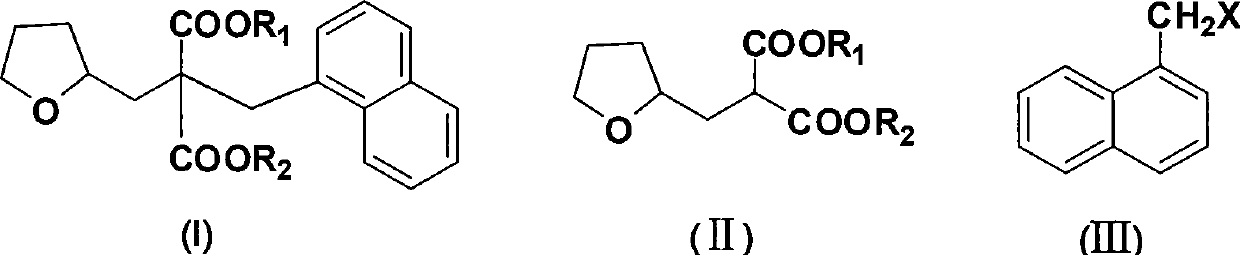
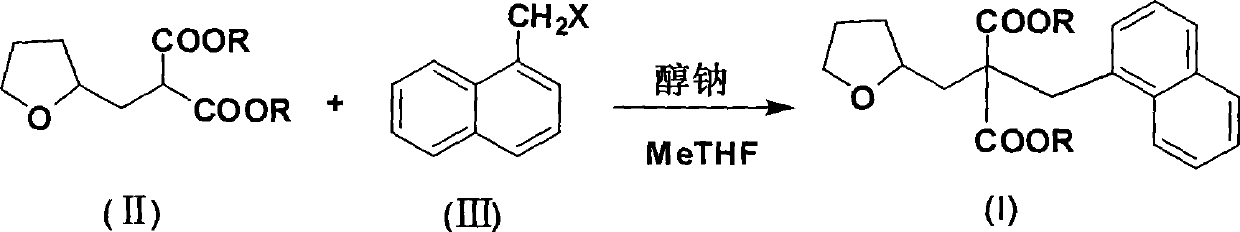
Synthetic method of diethyl naphthylmethyl-tetrahydrofurfurylmalonate
A technology of naphthylmethyl tetrahydrofurfuryl malonate diester and synthesis method, applied in directions such as organic chemistry, can solve problems such as difficulty in solvent recovery, increased production cost, troublesome processing, etc., and achieves less environmental pollution and high recovery rate , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The molar ratio of the feed materials is dimethyl tetrahydrofurfurylmalonate: sodium methoxide: 1-chloromethylnaphthalene = 1.0:1.0:0.9, and the temperature for dropping 1-chloromethylnaphthalene is 80°C.
[0028] Dimethyl tetrahydrofurfurylmalonate (40g, 0.185mol) and 2-methyltetrahydrofuran (200g, 233ml) were added into the reaction flask, and sodium methoxide (10g, 0.185mol) was added in batches at 20-45°C. ), heated and refluxed for 0.5 hours, then slowly added dropwise 1-chloromethylnaphthalene (29.4g, 0.167mol) at 80°C, refluxed for 1 hour, cooled to 20-45°C, added purified water and stirred for 10 minutes, static After settling, the organic layer was separated, and the aqueous layer was extracted with 50 mL of 2-methyltetrahydrofuran × 2. The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and 278.9 g of 2-methyltetrahydrofuran was recovered by rectification under normal pressure. The yield was 97.5%, and ...
Embodiment 2
[0030] The molar ratio of the feed materials is dimethyl tetrahydrofurfurylmalonate: sodium methoxide: 1-chloromethylnaphthalene = 1.0: 1.3: 1.0, and the temperature for dropping 1-chloromethylnaphthalene is 80°C.
[0031] Add dimethyl tetrahydrofurfurylmalonate (40g, 0.185mol) and 2-methyltetrahydrofuran (300g, 350ml) into the reaction flask, and add sodium methoxide (13g, 0.241mol) in batches at 20-45°C ), heated and refluxed for 0.5 hours, then slowly added dropwise 1-chloromethylnaphthalene (32.7g, 0.185mol) at 80°C, refluxed for 1 hour, cooled to 20-45°C, added purified water and stirred for 10 minutes, static After settling, the organic layer was separated, and the aqueous layer was extracted with 50 mL of 2-methyltetrahydrofuran × 2. The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and 375.5 g of 2-methyltetrahydrofuran was recovered by rectification under normal pressure. The yield was 97.3%, and 56.1 g of di...
Embodiment 3
[0033] The molar ratio of the feed materials is dimethyl tetrahydrofurfurylmalonate: sodium methoxide: 1-chloromethylnaphthalene = 1.0: 1.2: 1.0, and the temperature for dropping 1-chloromethylnaphthalene is 80°C.
[0034] Add dimethyl tetrahydrofurfurylmalonate (40g, 0.185mol) and 2-methyltetrahydrofuran (200g, 233ml) into the reaction flask, and add sodium methoxide (12g, 0.222mol) in batches at 20-45°C ), heated and refluxed for 0.5 hours, then slowly added dropwise 1-chloromethylnaphthalene (32.7g, 0.185mol) at 80°C, refluxed for 1 hour, cooled to 20-45°C, added purified water and stirred for 10 minutes, static After settling, the organic layer was separated, and the aqueous layer was extracted with 2-methyltetrahydrofuran (50mL×2). The yield was 97.9%, and 56.7 g of dimethyl naphthylmethyltetrahydrofurfurylmalonate was obtained, with a yield of 86.1% and a gas phase purity of 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com