Preparation of N-hydroxy diimide
A technology of hydroxyimide and hydroxylamine, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problem of easy decomposition and explosion, unstable hydroxylamine solution, and cannot be promoted and other problems, to achieve the effects of reducing production costs, mild reaction conditions, reaction yield and product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
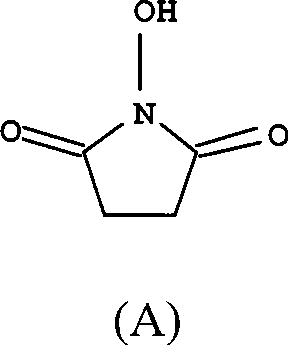
[0024] Add succinic anhydride (1mol), hydroxylamine hydrochloride (1mol), acetic anhydride (0.02mol), trifluoroacetic acid (0.02mol), pyridine (1mol) into a three-necked flask equipped with a stirrer, nitrogen inlet tube, and reflux condenser in sequence. ) and dioxane (400ml). Under the protection of nitrogen, heat up to 100°C, react for 6h, until no succinic anhydride is detected by thin layer chromatography, cool to 60°C, concentrate to dryness under reduced pressure, add 4000ml of ethyl acetate, stir, stand still, pour out the upper clear night , then extracted 4 times with ethyl acetate, each 2000ml, combined ethyl acetate solution, evaporated solvent under reduced pressure, recrystallized with ethyl acetate, decolorized with activated carbon, and obtained white solid, i.e. compound (A), yield 86% , melting point 97 ~ 98 ℃, purity > 99.5%.
Embodiment 2
[0026] Add succinic anhydride (1mol), hydroxylamine hydrochloride (1.05mol), acetic anhydride (0.04mol), trifluoroacetic acid (0.03mol), pyridine ( 1.05mol) and dioxane (400ml). Under the protection of nitrogen, heat up to 100°C, react for 6h, until no succinic anhydride is detected by thin layer chromatography, cool to 60°C, concentrate to dryness under reduced pressure, add 4000ml of ethyl acetate, stir, stand still, pour out the upper clear night , then extracted 4 times with ethyl acetate, each 2000ml, combined ethyl acetate solution, evaporated solvent under reduced pressure, recrystallized with ethyl acetate, activated carbon decolorization, obtained white solid, i.e. compound (A), yield 90% , melting point 97 ~ 98 ℃, purity > 99.5%.
Embodiment 3
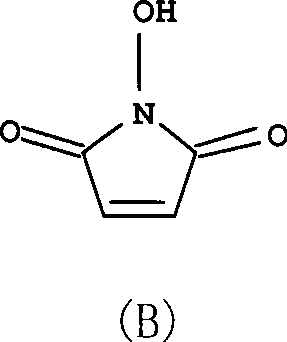
[0028] Add maleic anhydride (1mol), hydroxylamine sulfate (1.1mol), acetic anhydride (0.05mol), trifluoroacetic acid (0.05mol), triethyl Amine (1.25mol) and 2-methyltetrahydrofuran (400ml). Under the protection of nitrogen, heat up to 80°C, react for 6h, until there is no maleic anhydride detected by thin layer chromatography, cool to 50°C, concentrate to dryness under reduced pressure, add 4000ml of ethyl acetate, stir, stand still, pour out the upper clear night , extracted 4 times with ethyl acetate, each time 2000ml, combined ethyl acetate solution, evaporated solvent under reduced pressure, then recrystallized with ethyl acetate, activated carbon decolorization, obtained white solid, i.e. compound (B), yield 80% , melting point 148 ~ 149 ℃ (decomposition), purity> 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com