Method for enhancing ionization efficiency of peptide segment
An ionization and high-efficiency technology, applied in the field of biochemical analysis, can solve the problems of poor mass spectrometry detection, difficult removal of high-abundance protein suppression, increased sample loss, pollution, etc., to improve ionization efficiency and increase mass spectrometry detection sensitivity , Improve the effect of ionization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
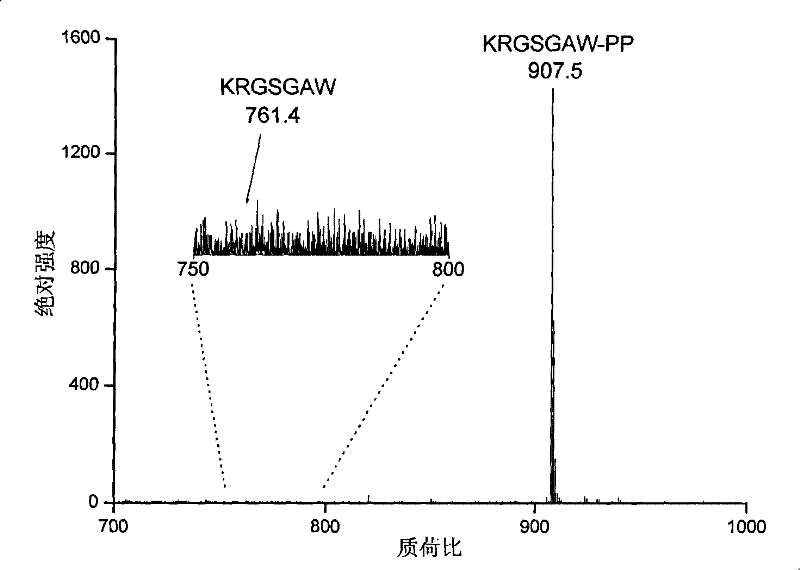
[0030] Example 1 verifies that the reaction efficiency of the derivatization reaction is close to 100%
[0031] 1-(2-pyrimidinyl)piperazine was diluted in N,N-dimethylformamide, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 1 -Hydroxy-7-azobenzotriazole was respectively dissolved in N,N-dimethylformamide to prepare a solution of 2 mg / ml. Add 6, 4 and 3 microliters of the above three reagents in sequence to 50 microliters of peptide KRGSGAW aqueous solution with a concentration of about 100 nanograms per microliter, and adjust the pH value to between 7.5 and 7.8 with 0.1% trifluoroacetic acid aqueous solution between. The above mixed system was suspended at room temperature for 30 seconds. It was then dried by centrifugation under vacuum to remove all solvents. The above-mentioned dried product was dissolved in an aqueous solution containing 50% acetonitrile and 0.1% trifluoroacetic acid for MALDI-MS detection, and the results showed that the derivatization...
Embodiment 2
[0032] Example 2 verifies that the reaction efficiency of the derivatization reaction is close to 100%
[0033] 1-(2-pyrimidinyl)piperazine was diluted in N,N-dimethylformamide, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 1 -Hydroxy-7-azobenzotriazole was respectively dissolved in N,N-dimethylformamide to prepare a solution of 2 mg / ml. Add 6, 4 and 3 microliters of the above three reagents in sequence to 50 microliters of peptide DRVYIHPF aqueous solution with a concentration of about 100 nanograms per microliter, and adjust the pH value to between 7.5 and 7.8 with 0.1% trifluoroacetic acid aqueous solution between. The above mixed system was suspended at room temperature for 30 seconds. It was then dried by centrifugation under vacuum to remove all solvents. The above dried product was dissolved in an aqueous solution containing 50% acetonitrile and 0.1% trifluoroacetic acid for MALDI-MS detection, and the efficiency of the two-site derivatization react...
Embodiment 3
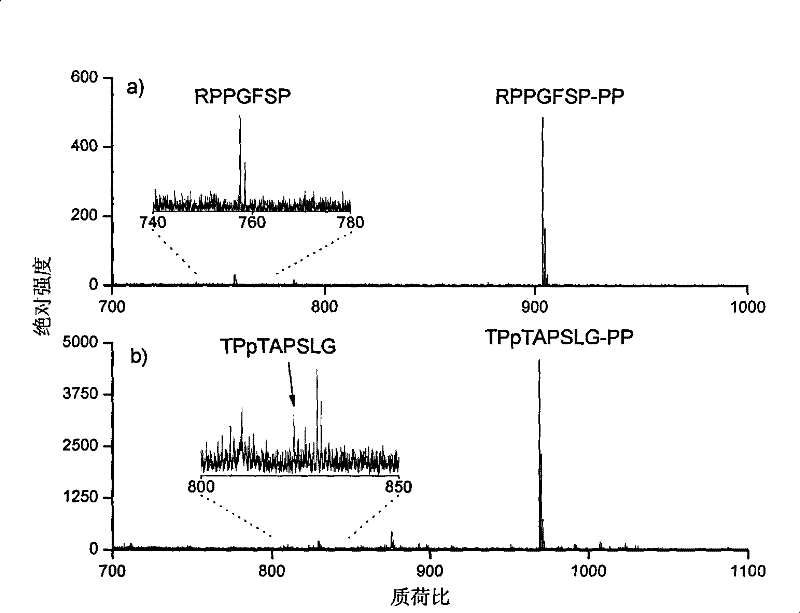
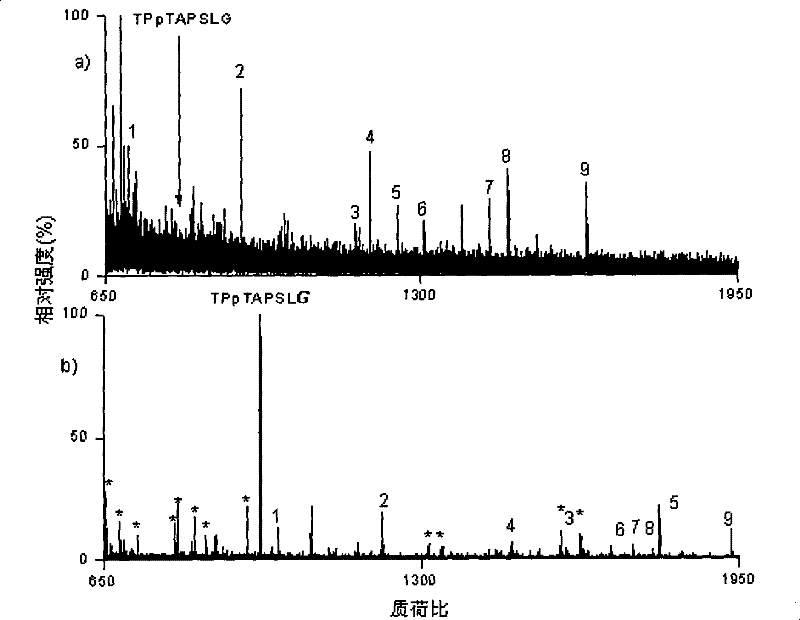
[0034] Example 3 The multiple of the ionization efficiency of the peptide segment RPGFSP after derivatization
[0035] 1-(2-pyrimidinyl)piperazine was diluted in N,N-dimethylformamide, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 1 -Hydroxy-7-azobenzotriazole was respectively dissolved in N,N-dimethylformamide to prepare a solution of 2 mg / ml. Add 6, 4 and 3 microliters of the above three reagents in sequence to 50 microliters of the peptide RPGGFSP aqueous solution with a concentration of about 100 nanograms per microliter, and adjust the pH value to between 7.5 and 7.8 with 0.1% trifluoroacetic acid aqueous solution between. The above mixed system was suspended at room temperature for 30 seconds. It was then dried by centrifugation under vacuum to remove all solvents. Add the same amount of underivatized peptides to each peptide derivative product, and dissolve the above products with an aqueous solution containing 50% acetonitrile and 0.1% trifluoroace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com