Process for synthesizing iron-nickel alloy nano particle catalyst for selective hydrogenation
A nanoparticle, iron-nickel alloy technology, applied in the field of synthesis of iron-nickel alloy nanoparticle catalysts for selective hydrogenation, can solve the problems of low decomposition temperature, toxic nickel carbonyl, inconvenient storage and transportation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
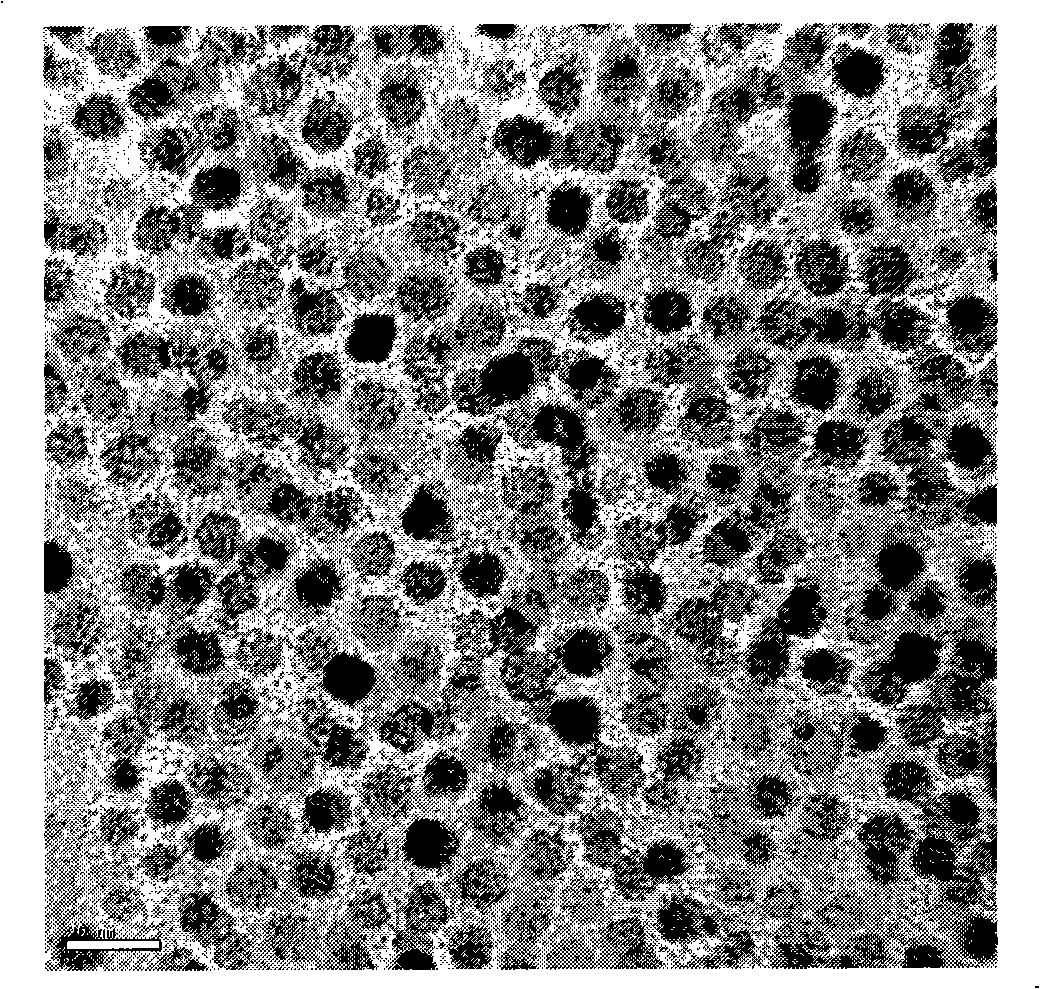
[0030] Put 2mmol of iron acetylacetonate, 2mmol of nickel acetylacetonate, 4ml of trioctylphosphine, 6ml of oleylamine, 3mmol of 1,2-hexadecanoyl alcohol, and 40ml of liquid paraffin into a 100ml stainless steel autoclave reactor, and pass it into a high-purity Nitrogen deoxygenation. Pass hydrogen gas (purity 99.9%) into the autoclave, fill the pressure to 4.0MPa, adjust the stirring speed to 500rpm, then raise the temperature to 300°C, and react for 3 hours. After the reaction was completed, the reactor was opened, and the reaction solution was taken out and placed in a 250 ml beaker. Add 40 milliliters of ethanol and 10 milliliters of petroleum ether (60-90° C.) into the beaker, stir well and place it on the surface of the permanent magnet. The liquid in the beaker will separate into two layers, remove the supernatant. Repeat the above steps 3-5 times, the black nanoparticles will be enriched at the bottom of the beaker. The obtained nanoparticles are vacuum-dried at 100...
Embodiment 2
[0032] 10 ml of p-chloronitrobenzene was dissolved in 30 ml of ethanol, the solution was added into an autoclave reactor, and then 0.2 g of iron-nickel alloy nanoparticles was added into the autoclave. Nitrogen was introduced into the reactor to replace it to remove oxygen, and then hydrogen was introduced. The initial hydrogen pressure is 4.0MPa.
[0033] The temperature of the reactor was raised to 100°C, and the temperature was kept constant for one hour. After the reaction is over, after the reactor body is cooled, hydrogen gas is released to open the reactor, and the black liquid is taken out into a beaker. The beaker is placed on the surface of the permanent magnet, and after a few minutes, the magnetic nano-catalyst particles are concentrated at the bottom of the beaker. The clear solution was removed for chromatographic analysis.
[0034]
[0035] The results of chromatographic analysis showed that p-chloronitrobenzene was converted into p-chloroaniline with a co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com