Pirarubicin or pirarubicin hydrochloride lipid nano granule and preparation method thereof
A technology of pirarubicin hydrochloride and lipid nanoparticles, which is applied in liposome delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of large toxic side effects and poor stability, and achieve reduced toxicity, Effect of reducing toxicity and increasing concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
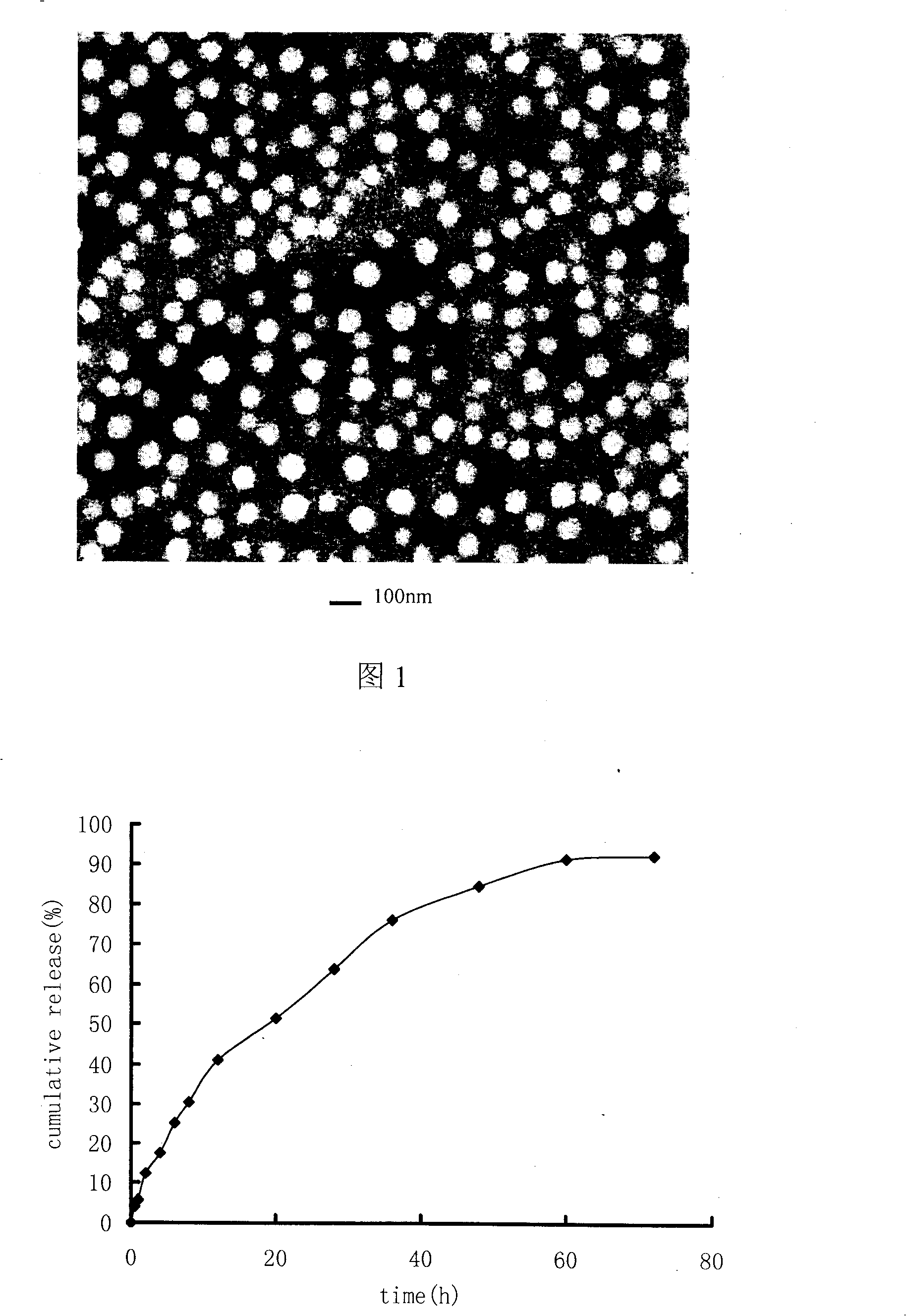
[0033] Get the pirarubicin of 20mg, 100mg stearic acid and dissolve in 20ml dichloromethane, as organic phase, 100mg poloxamer 108 is added in 50ml water as water phase, organic phase is added in the water phase and carries out high-speed shearing ( 6000rpm, 100s) emulsification, and then use a rotary evaporator to remove the organic solvent in a water bath at 60°C, and emulsify 5 times under high pressure at 300 atmospheres to obtain a lipid nanoparticle dispersion system. Its average particle size is 120nm, accounting for 80%, all particles are below 250nm, and the particle size distribution is narrow, indicating that the size of lipid nanoparticles is relatively uniform. The nanoparticle suspension can be stable for several days at room temperature and stable at 4°C At 12 months, no precipitation or degradation of pirarubicin was observed during storage.
Embodiment 2
[0035] Dissolve 10mg of pirarubicin and 800mg of lecithin in 60ml of chloroform as the organic phase, add 250mg of Benze 78 into 80ml of water as the water phase, add the organic phase to the water phase for high-speed shear (10000rpm, 160s) emulsification , and then use a rotary evaporator to remove the organic solvent in a water bath at 65° C., and perform high-pressure emulsification at 500 atmospheres for 10 times to obtain a lipid nanoparticle dispersion system. Its average particle size is 80nm, accounting for 80%, all particles are below 140nm, and the particle size distribution is narrow, indicating that the size of lipid nanoparticles is relatively uniform. The nanoparticle suspension can be stable for several days at room temperature and stable at 4°C 12 months.
Embodiment 3
[0037]Take 15 mg of pirarubicin and 600 mg of glyceryl monostearate dissolved in 50 ml of cyclohexane as the organic phase, add 200 mg of sodium cholate into 100 ml of water as the water phase, add the organic phase to the water phase for high-speed shearing (6000rpm, 50s) emulsification, and then remove the organic solvent with a rotary evaporator in a water bath at 45°C, and emulsify 4 times at 200 atmospheres of pressure to obtain a lipid nanoparticle dispersion system. Its average particle size is 300nm, accounting for 70%, and all particles are below 500nm. The nanoparticle suspension is stable for several days at room temperature and 12 months at 4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com