Hafnium compound, hafnium thin film-forming material and method for forming hafnium thin film
An amine compound and compound technology, which is applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, metal material coating processes, etc., can solve the problem of difficult gasification, poor stability, and difficulty in supplying stable gases and other problems, to achieve the effect of stable supply and easy gasification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] [The novel compound CpHf(NMe 2 ) 3 Synthesis]
[0055] Under a nitrogen atmosphere, 29 g of tetrakis(dimethylamino)hafnium and 5.3 g of cyclopentadiene were stirred in 70 mL of benzene for 1 hour. After heating the solution to reflux for 2 hours, benzene was distilled off under reduced pressure. The remaining yellow liquid was subjected to 0.1 torr vacuum distillation at 70-80° C. to obtain 25 g of yellow liquid.
[0056] The yellow liquid boiled at 75°C / 0.2 Torr.
[0057] In addition, the results and spectrum of NMR measurement showed the following resonance lines.
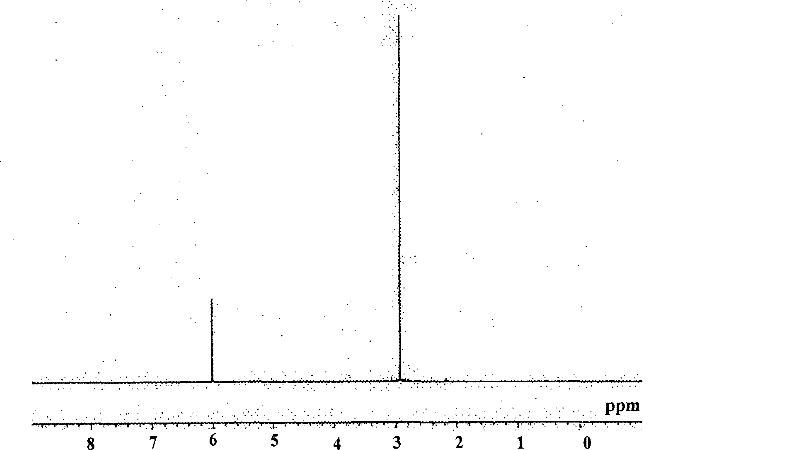
[0058] 1 H-NMR (C 6 D. 6 ): 3.0(s, 18H), 6.0(s, 5H)
[0059] What needs to be explained is that, figure 1 is the NMR spectrum of cyclopentadienyl tris(dimethylamino)hafnium.
[0060] And, judging from the above reaction form and NMR spectrum: the obtained yellow liquid is cyclopentadienyl tri(dimethylamino) hafnium [CpHf(NMe 2 ) 3 ].
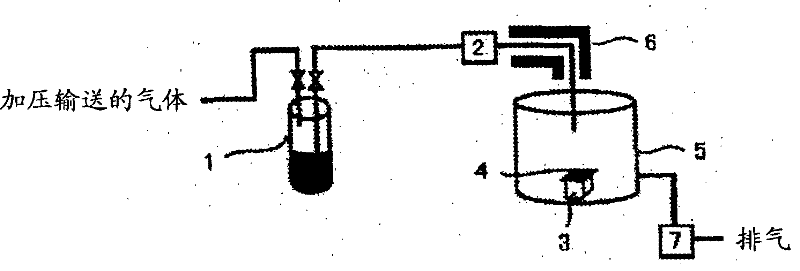
[0061] Next, 150 g of CpHf(NMe 2 ) 3 It was charged int...
Embodiment 2
[0071] [Novel compound (MeCp)Hf(NMe 2 ) 3 Synthesis]
[0072] Under a nitrogen atmosphere, 10 g of tetrakis(dimethylamino)hafnium and 2 g of methylcyclopentadiene were stirred in 35 mL of benzene for 1 hour. After heating the solution to reflux for 2 hours, benzene was distilled off under reduced pressure. The remaining yellow liquid was subjected to 0.1 torr vacuum distillation at 70-80° C. to obtain 11 g of yellow liquid.
[0073] The yellow liquid has a boiling point of 72°C / 0.1 Torr.
[0074] In addition, the results and spectrum of NMR measurement showed the following resonance lines.
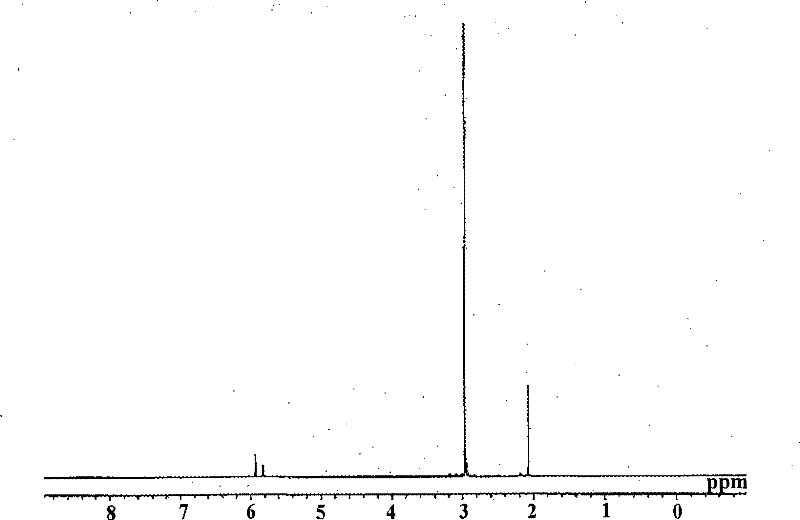
[0075] 1 H-NMR (C 6 D. 6 ): 2.1(s, 3H), 3.0(s, 18H), 5.8(m, 2H), 5.9(m, 2H)
[0076] What needs to be explained is that, image 3 is the NMR spectrum of methylcyclopentadienyl tris(dimethylamino)hafnium.
[0077] Moreover, judging from the above reaction form and NMR spectra: the obtained yellow liquid is methylcyclopentadienyl tris(dimethylamino)hafnium [(MeCp)Hf(NMe 2 ) 3 ]. ...
Embodiment 3
[0085] [Novel compound (EtCp)Hf(NMe 2 ) 3 Synthesis]
[0086] Under a nitrogen atmosphere, 25 g of tetrakis(dimethylamino)hafnium and 7 g of ethylcyclopentadiene were stirred in 60 mL of benzene for 1 hour. After heating the solution to reflux for 2 hours, benzene was distilled off under reduced pressure. The remaining yellow liquid was subjected to 0.1 torr vacuum distillation at 70-80° C. to obtain 19 g of yellow liquid.
[0087] The yellow liquid boiled at 78°C / 0.2 Torr.
[0088] In addition, the results and spectrum of NMR measurement showed the following resonance lines.
[0089] 1 H-NMR (C 6 D. 6 ): 1.1(t, 3H), 2.5(q, 2H), 3.0(s, 18H), 5.9(m, 2H), 6.0(m, 2H)
[0090] What needs to be explained is that, Figure 4 is the NMR spectrum of ethylcyclopentadienyl tris(dimethylamino)hafnium.
[0091] Moreover, judging from the above reaction form and NMR spectrum: the obtained yellow liquid is ethylcyclopentadienyl tri(dimethylamino) hafnium [(EtCp)Hf(NMe 2 ) 3 ].
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com