Secondary fermentation method natural medicinal preparation, preparation and use
A secondary fermentation method, a technology of natural medicines, applied in the field of multi-component natural medicine preparations, can solve problems such as the unreported process of natural antibacterial medicine preparations, and achieve significant social benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
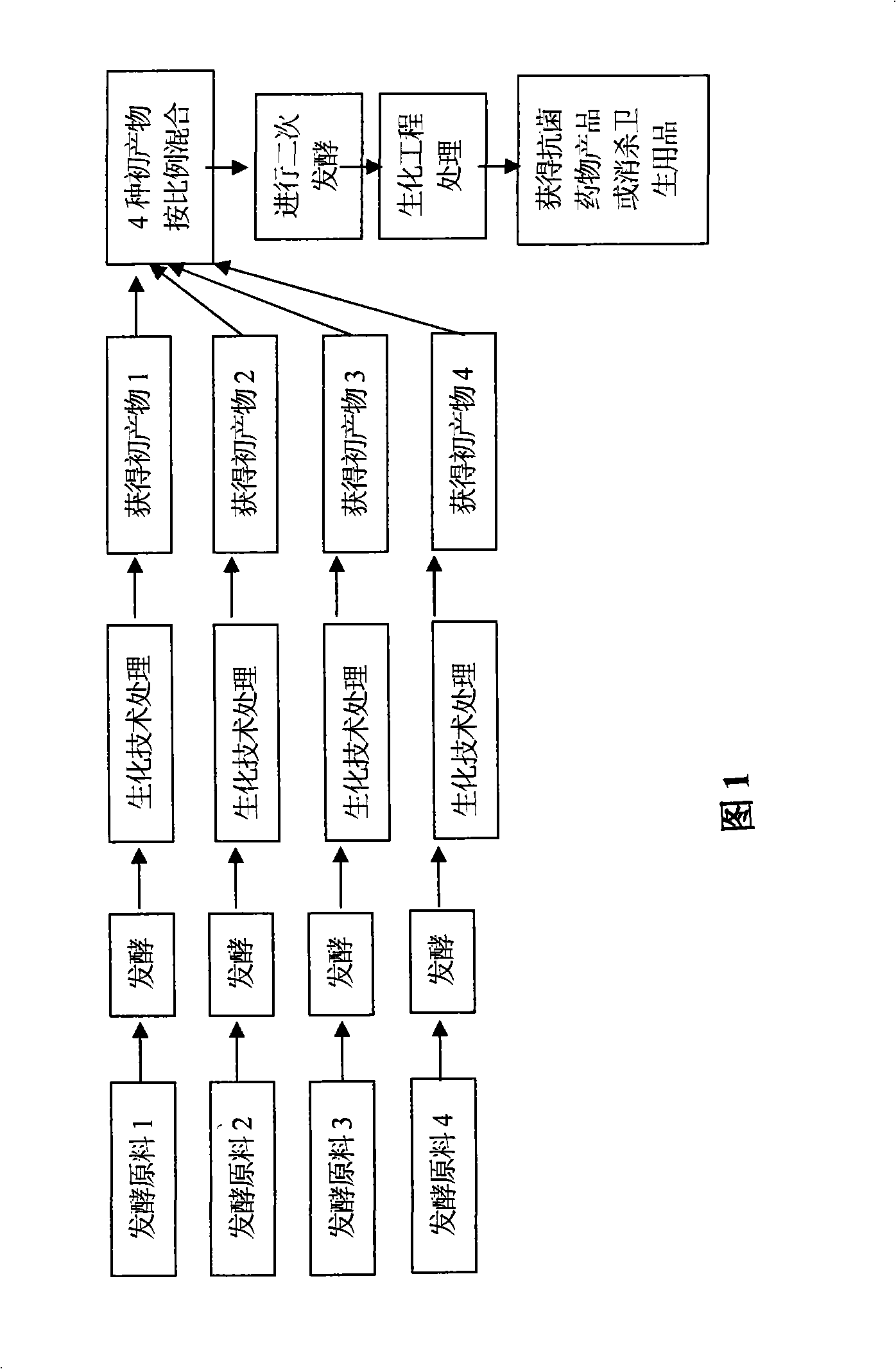
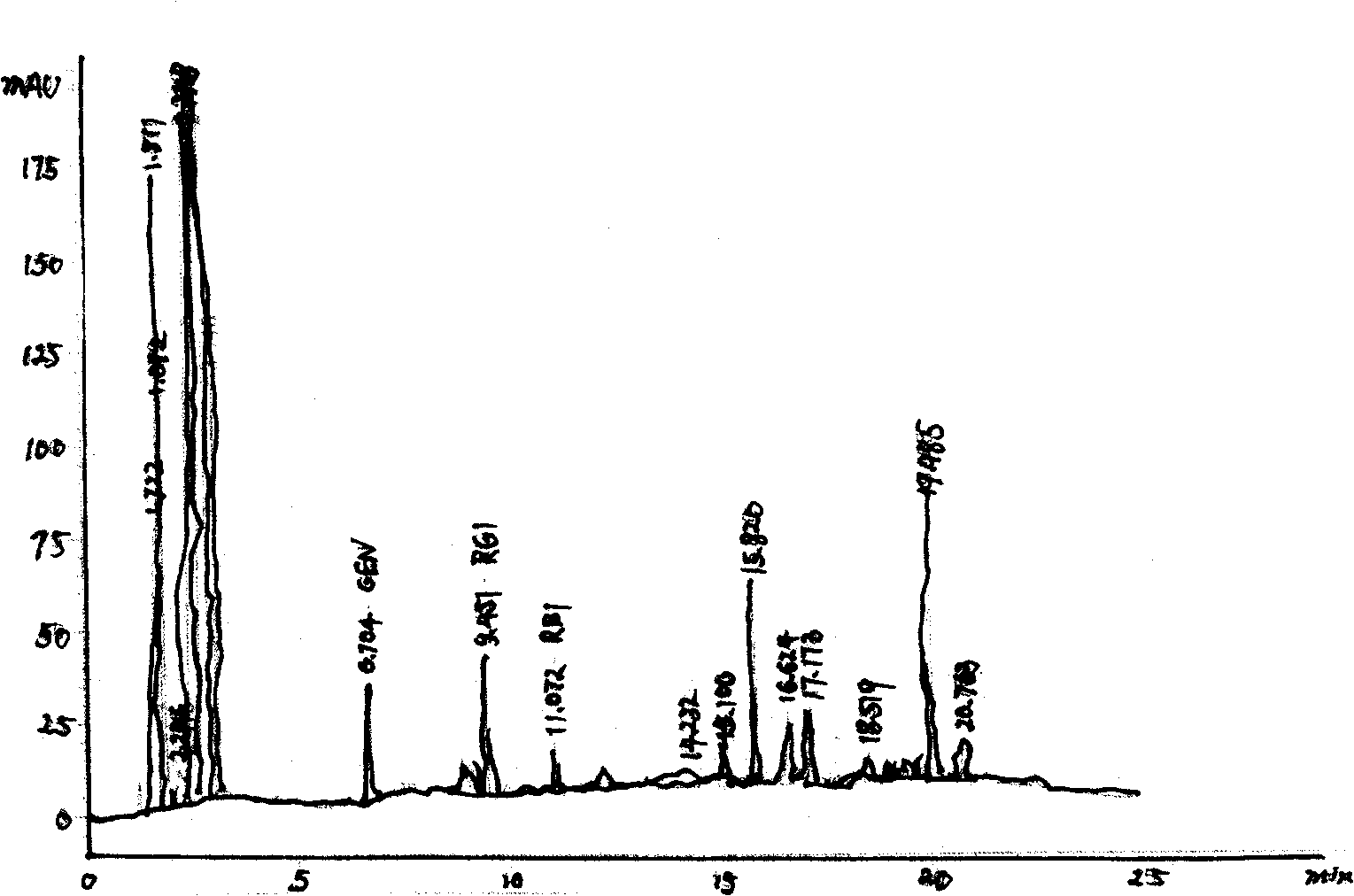
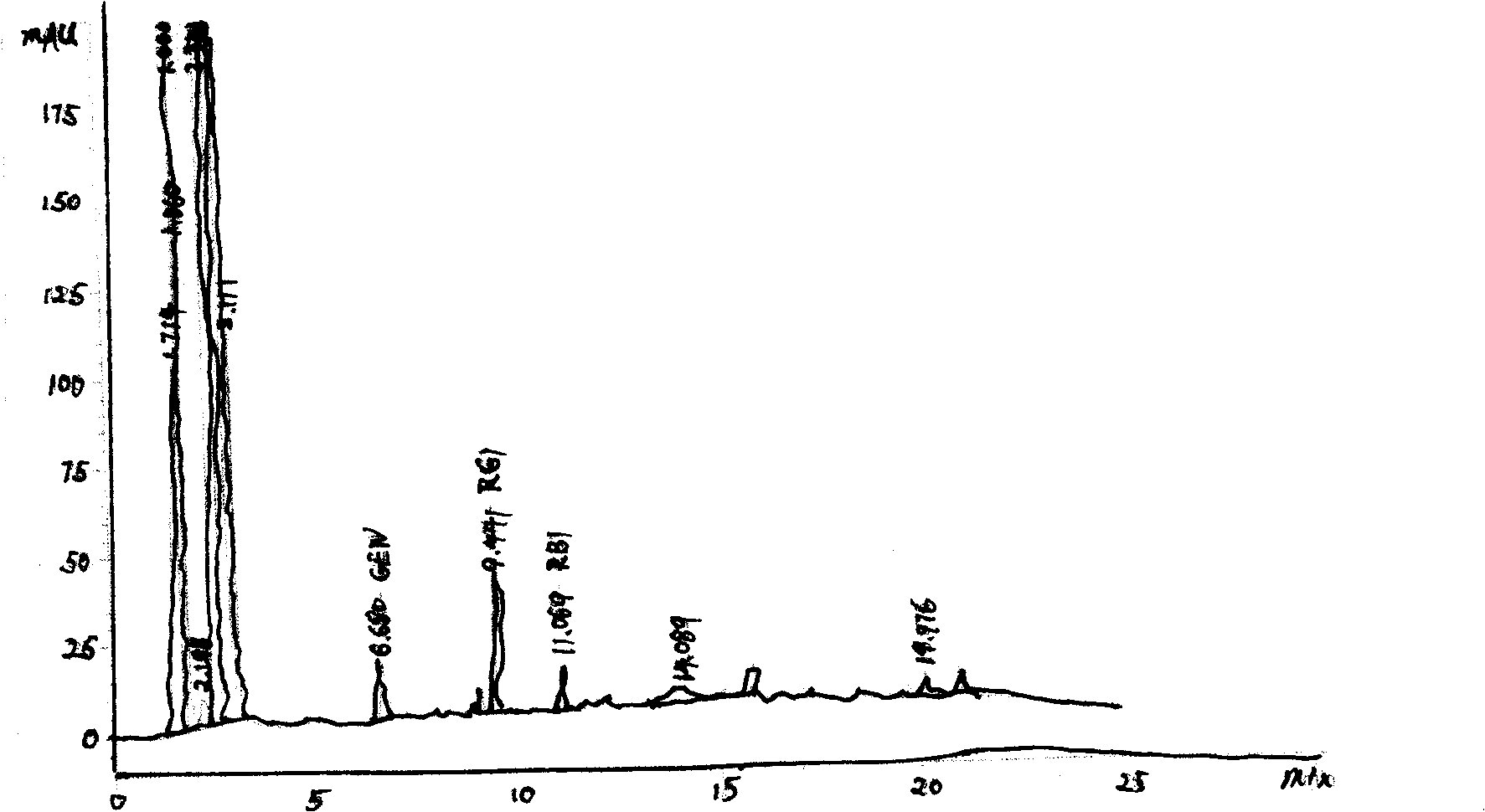
Image
Examples
Embodiment 1
[0025] Strain preparation: The production strain is Ganoderma lucidum (Leyss.ex Fr.)
[0026] Traits: Ganoderma lucidum commonly referred to refers to the fruit body of Ganoderma lucidum. In the present invention, the mycelium of Ganoderma lucidum is mainly used as a strain for fermentation. Mycelium culture characteristics and microscopic examination: The strains are inoculated on the potato culture slant, the temperature is 24-26℃, and the cultivation time is 120 hours to grow colorless or nearly white flocculent mycelium, or do a single plate colony culture, colony Irregular, colorless or nearly white flocculent mycelium, with radial edges.
[0027] Inoculate the above-mentioned strains in potato culture medium, culture at 24-35℃, shaker 180-220 times / min, pH value is natural, culture for 5-7 days, make bacterial suspension, as the first time and Strains for the second fermentation (bacteria suspension).
[0028] The first fermentation: Weigh 100g of gentian grass, mulberry bar...
Embodiment 2
[0031] Example 2: The four fermentation broths were mixed in a ratio of 1:3:3:1, propylene glycol used 10%; purified water used 70%, and the others were the same as in Example 1, and will not be repeated.
Embodiment 3
[0032] Example 3: Four fermentation broths were mixed at a ratio of 1:2.2:2.2:1; propylene glycol was 8%; purified water was 73%. Others are the same as in Example 1, and will not be repeated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com