Temperature-induced gelatinizing-in-situ triblock copolymer, preparation method and applications
An in-situ gel and temperature-induced technology, applied in the field of vascular embolization materials, can solve the problems of easy precipitation, dehydration and collapse of polymer chain segments, and the inability to pre-design the molecular structure of copolymer materials, etc., to achieve fast transformation and good biological compatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
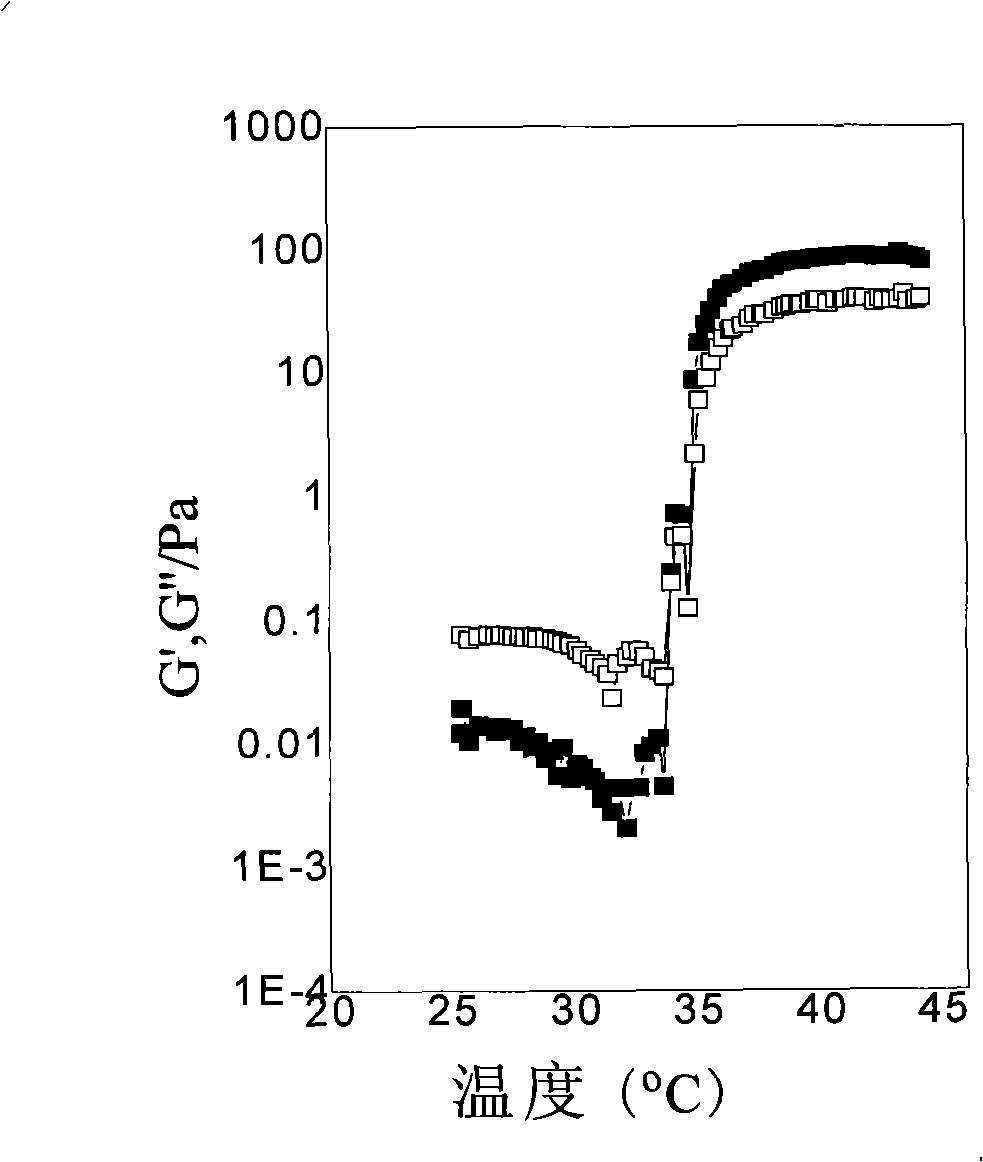
Examples
Embodiment 1
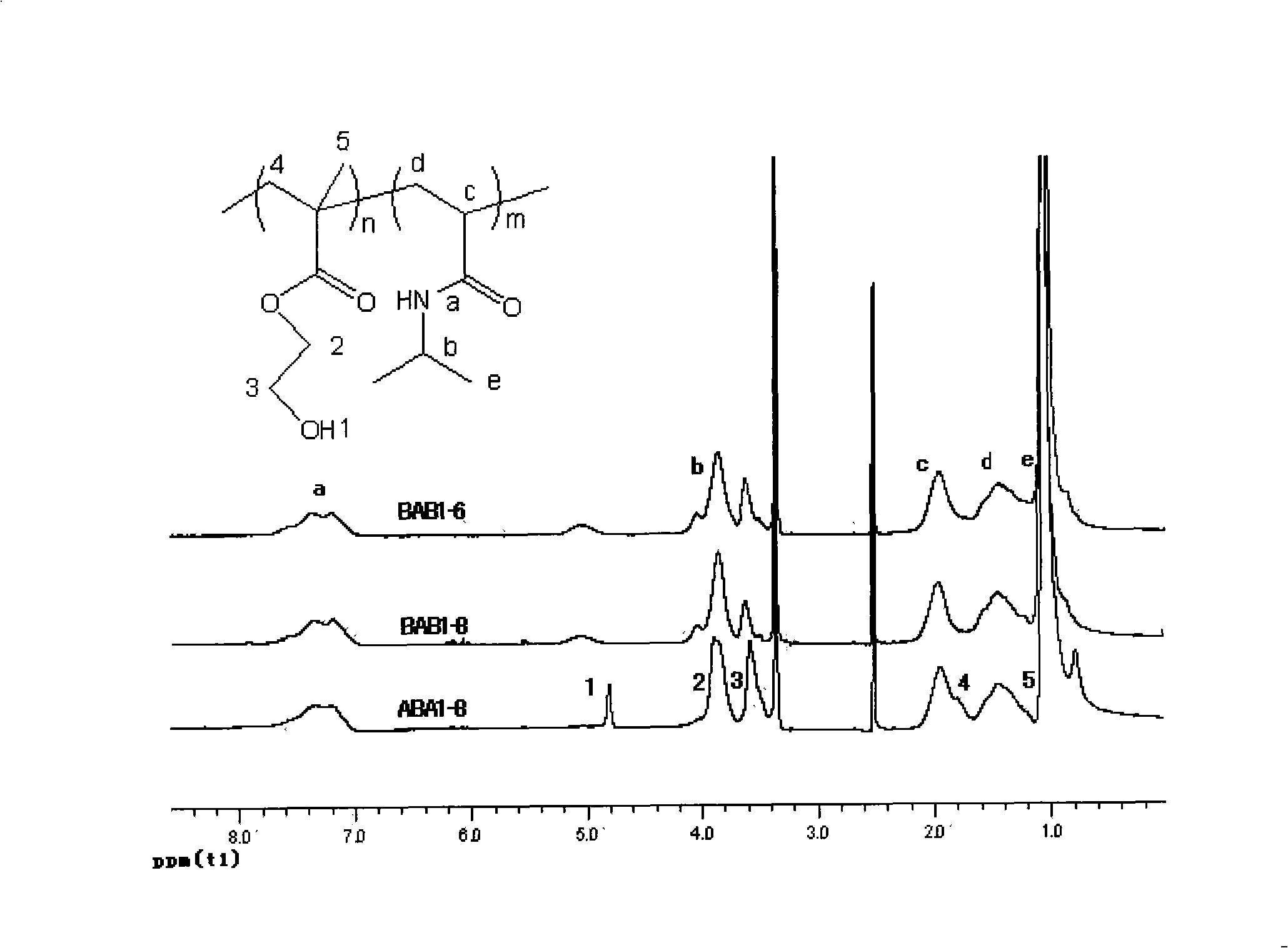
[0027] Add the bifunctional initiator diethyl m-2,5-dibromoadipate (9.1mg, 0.025mmol), the catalyst cuprous chloride (4.9mg, 0.05mmol) and 2mL of methanol into the Schleck tube, and use liquid Nitrogen freezing (-198°C), degassing, and then thawing the system at 25°C while blowing nitrogen, repeated freezing and thawing three times in order to remove oxygen in the reaction system. The monomer isopropylacrylamide (NIPAAm) (1.3578g, 11.998mmol, theoretical degree of polymerization: 480) and the ligand 1,4,8,11-tetramethyl-1,4,8,11-tetrazaza Cyclotetradecane (12.8 mg, 0.050 mmol) was dissolved in 6 mL of methanol and transferred to the reaction vessel with a syringe under nitrogen protection. After the reaction mixture was repeatedly frozen and thawed three times, it was returned to room temperature (25° C.), and the reaction vessel was vacuum-sealed. After reacting for 6 hours, nitrogen gas with a purity of 99.99% was passed into the reaction system. Dissolve 2-hydroxyethyl met...
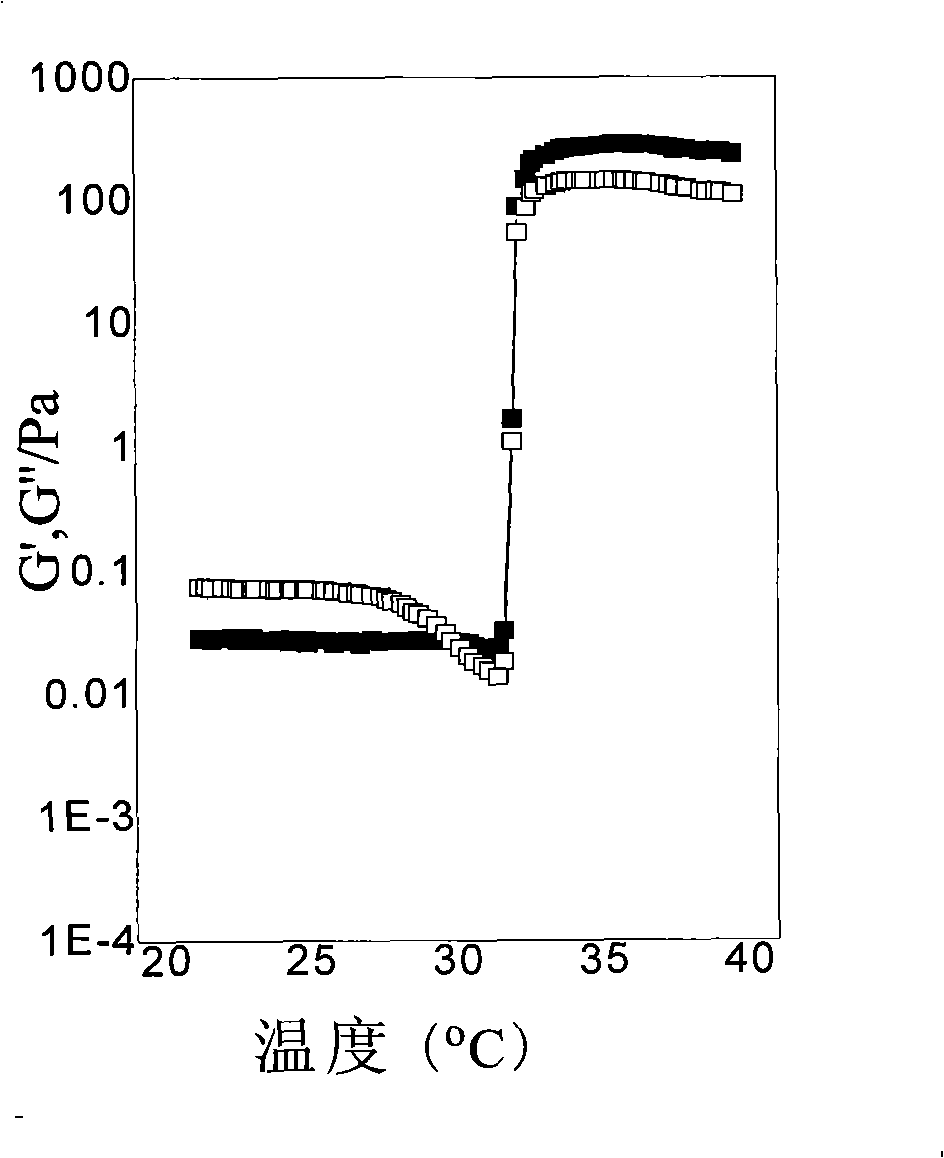
Embodiment 2
[0032] Add the bifunctional initiator diethyl m-2,5-dibromoadipate (9.1mg, 0.025mmol), the catalyst cuprous chloride (4.9mg, 0.05mmol) and 2mL of methanol into the Schleck tube, and use liquid Nitrogen freezing (-198°C), degassing, and then dissolving with nitrogen gas, repeated freezing and thawing three times in order to remove oxygen in the reaction system. The monomer isopropylacrylamide (NIPAAm) (1.8103g, 16mmol, theoretical degree of polymerization: 640) and the ligand 1,4,8,11-tetramethyl-1,4,8,11-tetrazazacyclo Tetradecane (12.8 mg, 0.050 mmol) was dissolved in 6 mL of methanol and transferred to the reaction vessel with a syringe under nitrogen protection. After the reaction mixture was repeatedly frozen and thawed three times, it was returned to room temperature, vacuum-sealed, and after 6 hours of magnetic stirring reaction, 99.99% nitrogen was passed into the reaction system, and 2ml of 2-hydroxyethyl methacrylate (HEMA) (0.2603 g, 2.0mmol, the theoretical polymer...
Embodiment 3
[0037] Diethyl m-2,5-dibromoadipate (9.1mg, 0.025mmol) and cuprous chloride (12.8mg, 0.050mmol) were used to add the bifunctional initiator into the Schleck tube, and frozen with liquid nitrogen (- 198°C), degassed, and then dissolved in nitrogen, so repeated freezing and thawing three times to remove oxygen in the reaction system. The monomer 2-hydroxyethyl methacrylate (HEMA) (0.2603g, 2.000mmol, theoretical degree of polymerization: 80) and the ligand 1,4,8,11-tetramethyl-1,4,8,11 - Tetrazaazacyclotetradecane (12.8 mg, 0.050 mmol) was dissolved in 6 mL of methanol at 25° C. in methanol and transferred to the reaction vessel with a syringe under nitrogen protection. After the reaction mixture was repeatedly frozen and thawed three times, it was returned to room temperature, and the reaction vessel was vacuum-sealed. After 6 hours of magnetic stirring reaction, 99.99% nitrogen was passed into the reaction system, and 6ml of isopropylacrylamide (NIPAAm) (1.810g, 16 mmol, each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com