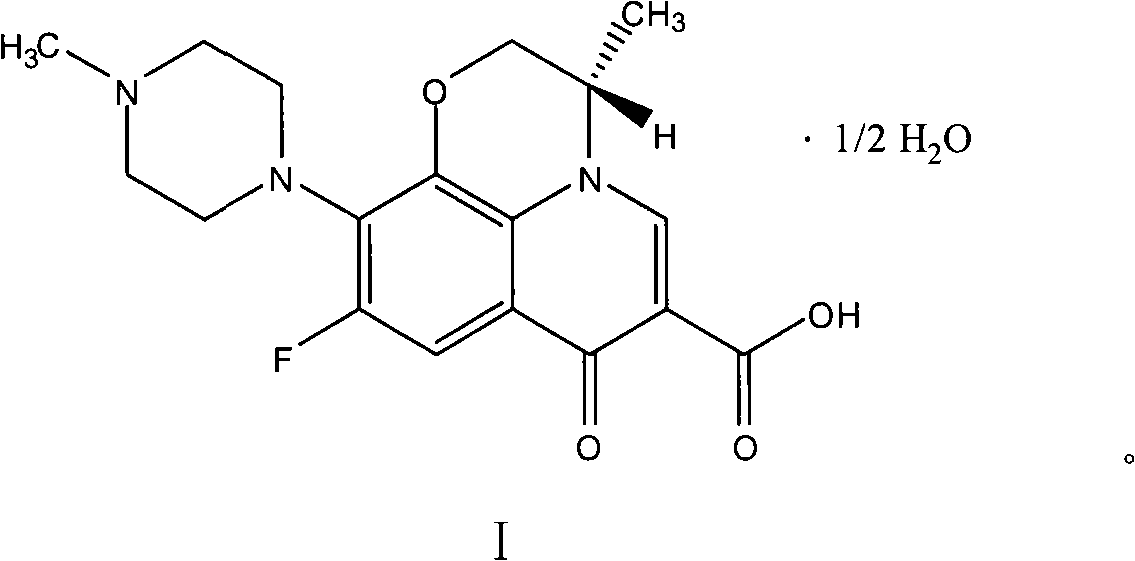
Process for preparing hemihydrate of levofloxacin
A technology for preparing levofloxacin and its preparation technology, which is applied in the field of preparation technology of levofloxacin hemihydrate, which can solve the problems of unfavorable quality and yield of levofloxacin hemihydrate finished product, complicated operation procedures, difficult microbial degradation, etc., and achieve mild polarity, color and luster Good, good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 n-Butanol: Water (90:10, w / w)
[0022] 50 grams of levofloxacin (0.1778 moles), 36 grams of N-methylpiperazine (0.3594 moles) were dropped into 1000 milliliters of reaction flasks equipped with a reflux device, then 500 g of DMF was added, stirred, heated and heated at 90 ~95°C heat preservation reaction for 6 hours. After the reaction is complete, recover the solvent under reduced pressure to dryness, add 400 ml of water to the residue in the bottle, stir at 60-65°C until the system is basically clear, add 1 g of activated carbon, keep warm for 0.5 hours to decolorize, filter, and add 500 ml of water to the filtrate Chloroform, stirred and extracted for 1 hour, the water layer was adjusted to pH 7.0-8.0 with liquid caustic soda, the extracted oil layer was stirred and washed with 300 ml of water for 15 minutes, left to stand for 1 hour, the organic layer of chloroform was separated, and heated and recovered to dryness to obtain Crude levofloxacin. Add 450 g...
Embodiment 2
[0023] Example 2 Isobutanol: water (90:10, w / w)
[0024] 50 grams of levofloxacin (0.1778 moles), 45 grams of N-methylpiperazine (0.4493 moles) were dropped into 1000 milliliters of reaction flasks equipped with a reflux device, then 450 g of DMSO was added, stirred, heated and heated at 100 ~105°C heat preservation reaction for 4 hours. After the reaction is complete, recover the solvent under reduced pressure to dryness, add 320 ml of water to the residue in the bottle, stir at 70-75°C until the system is basically clear, add 1.5 g of activated carbon, keep warm for 0.5 hours to decolorize, filter, and add 500 ml of water to the filtrate Dichloromethane, stirred and extracted for 1 hour, the water layer was adjusted to pH 7.0-8.0 with liquid alkali, the extracted oil layer was stirred and washed with 350 ml of water for 15 minutes, left to stand for 1 hour, the organic layer of dichloromethane was separated, and heated and recovered to dryness to obtain Crude levofloxacin. ...
Embodiment 3
[0025] Example 3 tert-butanol: water (90:10, w / w)
[0026]50 grams of levofloxacin (0.1778 moles) and 60 grams of N-methylpiperazine (0.5990 moles) were dropped into 1000 milliliters of reaction flasks equipped with a reflux device, then 550 g of DMSO was added, stirred, heated and heated at 100 ~105°C heat preservation reaction for 3.5 hours. After the reaction is complete, recover the solvent under reduced pressure to dryness, add 320 ml of water to the residue in the bottle, stir at 70-75°C until the system is basically clear, add 1.5 g of activated carbon, keep warm for 0.5 hours to decolorize, filter, and add 600 ml of water to the filtrate Toluene, stirred and extracted for 1 hour, the water layer was adjusted to pH 7.0-8.0 with liquid alkali, the extracted oil layer was stirred and washed with 400 ml of water for 15 minutes, left to stand for 1 hour, the toluene organic layer was separated, heated and recovered to dryness, and the crude product of levofloxacin was obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com