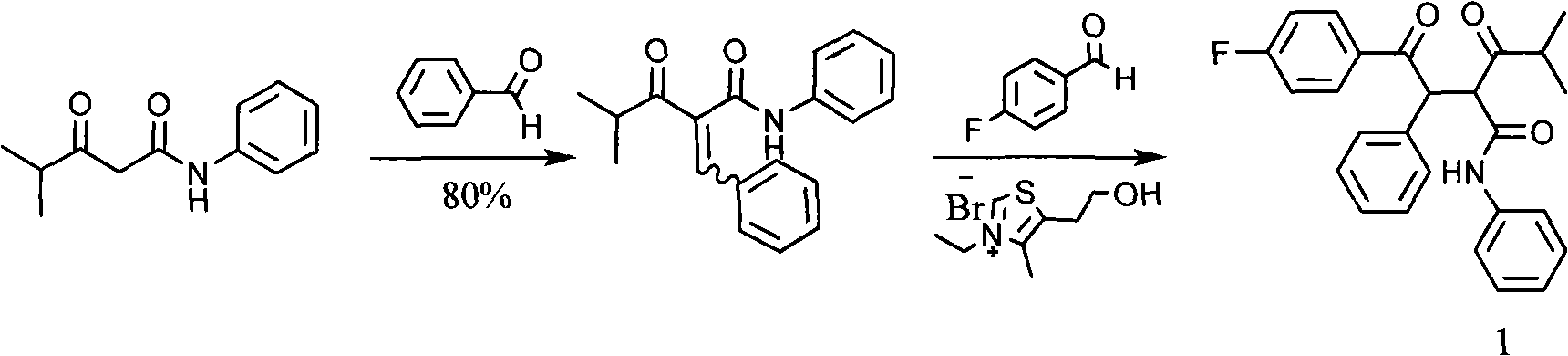
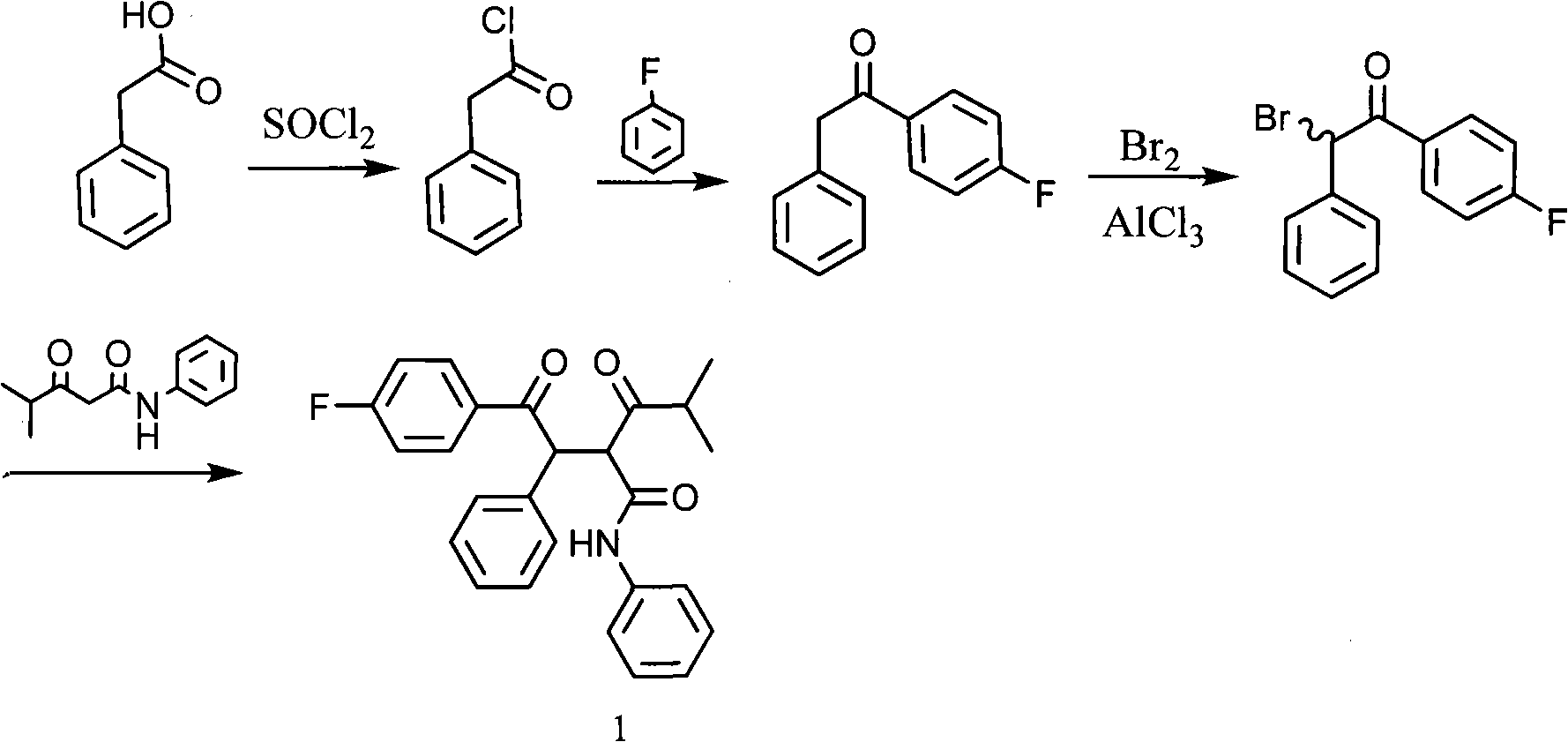
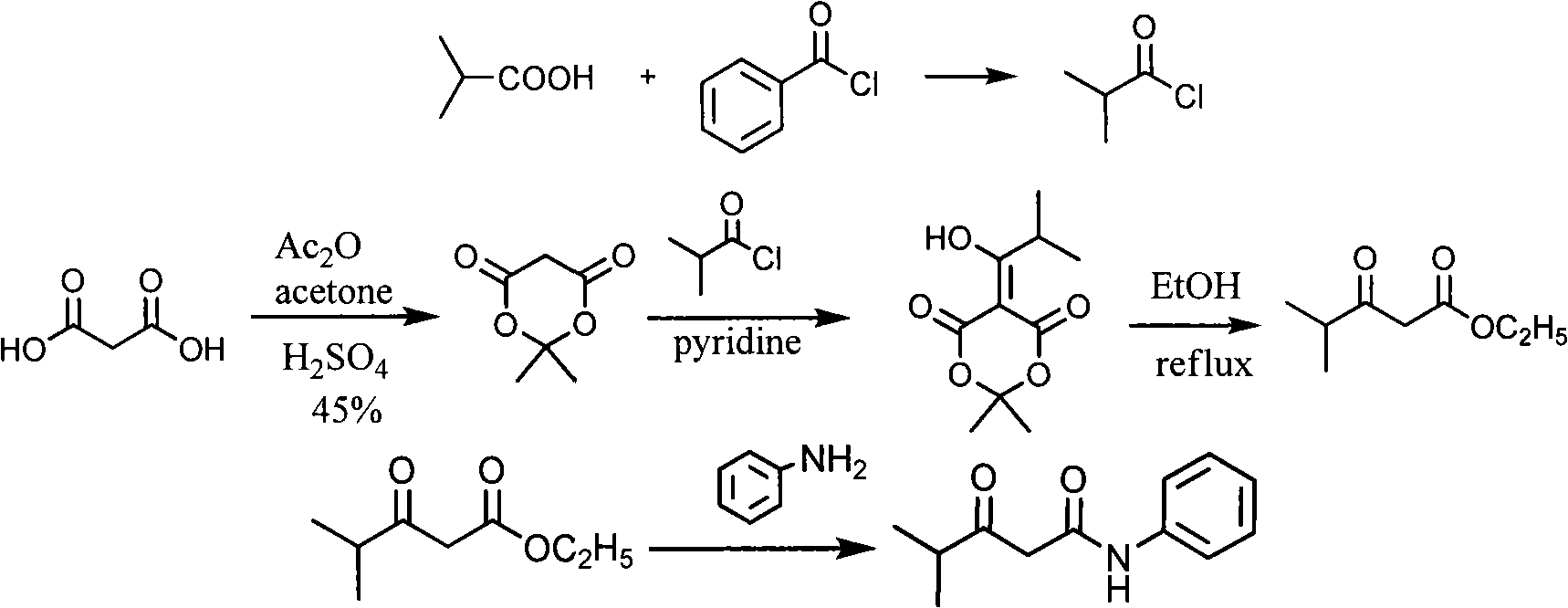
New synthetic method for (earth)4-fluor-alpha-(2-methyl-1-oxypropyl )-gamma-oxo-N, beta-diphenyl benzene butanamide
A technology of diphenylbenzamide and oxypropyl is applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of high industrialization cost, low yield of isobutyrylacetanilide, and raw materials. It is more expensive and other problems to achieve the effects of low production cost, improved total yield and short process route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Preparation of 4-fluorophenyl benzyl ketone (2)
[0018] 15.5g (0.10mol) of phenylacetyl chloride was dissolved in 30mL of fluorobenzene for later use.
[0019] 16g (0.12mol) AlCl 3 and 70mL of fluorobenzene were added to the reaction flask, and the fluorobenzene solution of phenylacetyl chloride was added dropwise under cooling in an ice bath, and the dropwise addition was completed in about 30 minutes. The reaction solution was stirred at -10 to 0°C for 3 hours, raised to room temperature, poured into 100 g of crushed ice and 30 mL of concentrated hydrochloric acid, separated to remove the water layer, washed the organic layer with 50 mL×3 water, dried over anhydrous sodium sulfate, and distilled off the fluorobenzene. The resulting solid was recrystallized from absolute ethanol to obtain 19.3 g of the product, with a yield of 90%. The melting point is 76-78°C.
Embodiment 2
[0020] Embodiment 2: Preparation of α-chloro-4-fluorophenyl benzyl ketone (3)
[0021] Compound (2) 21.4g (0.1mol) was dissolved in 100mL of dichloromethane, and chlorine gas was introduced at room temperature. After the detection was complete, the chlorine gas was stopped, washed with 50mL×3 water, the organic layer was dried over anhydrous sodium sulfate, and the dichloromethane was evaporated. The obtained crude product can be directly used in the next reaction.
Embodiment 3
[0022] Embodiment 3: the preparation of isobutyrylacetanilide (4)
[0023] In a 500mL three-necked flask, add 30g (0.33mol) dimethyl carbonate and 80mL toluene, add 11.2g (0.47mol) sodium hydrogen under stirring, heat to 70°C, add dropwise 10.4g (0.12mol) methyl isopropyl ketone, The dropwise addition was completed in about 1 hour, and then reacted for 4 hours after the dropwise addition. Under cooling, 50 mL of acetic acid was added dropwise, washed with 100 mL×3 water, dried over anhydrous sodium sulfate, evaporated toluene, and distilled under reduced pressure to obtain 18.1 g of methyl isobutyrylacetate with a yield of 95%.
[0024] In a 100mL three-necked flask, add 50mL toluene and 18.1g methyl isobutyryloacetate, add 10.7g (0.11mol) aniline dropwise under reflux, continue to react for 2 hours after the dropwise addition, cool to room temperature, wash with 3×100mL 10% hydrochloric acid , the organic layer was dried over anhydrous sodium sulfate, the toluene was distill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com