Dual sustained-release anticancer injection
A sustained-release injection and double-sustained-release technology, which is applied in the direction of antineoplastic drugs, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the complex implantation process, the degradation and degeneration of anticancer active ingredients, and the inability to effectively remove tumor cells And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
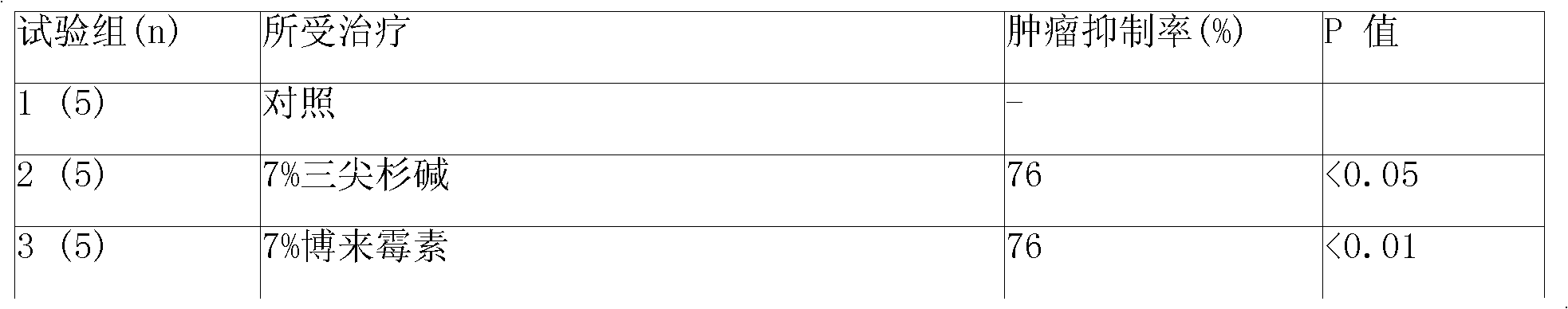
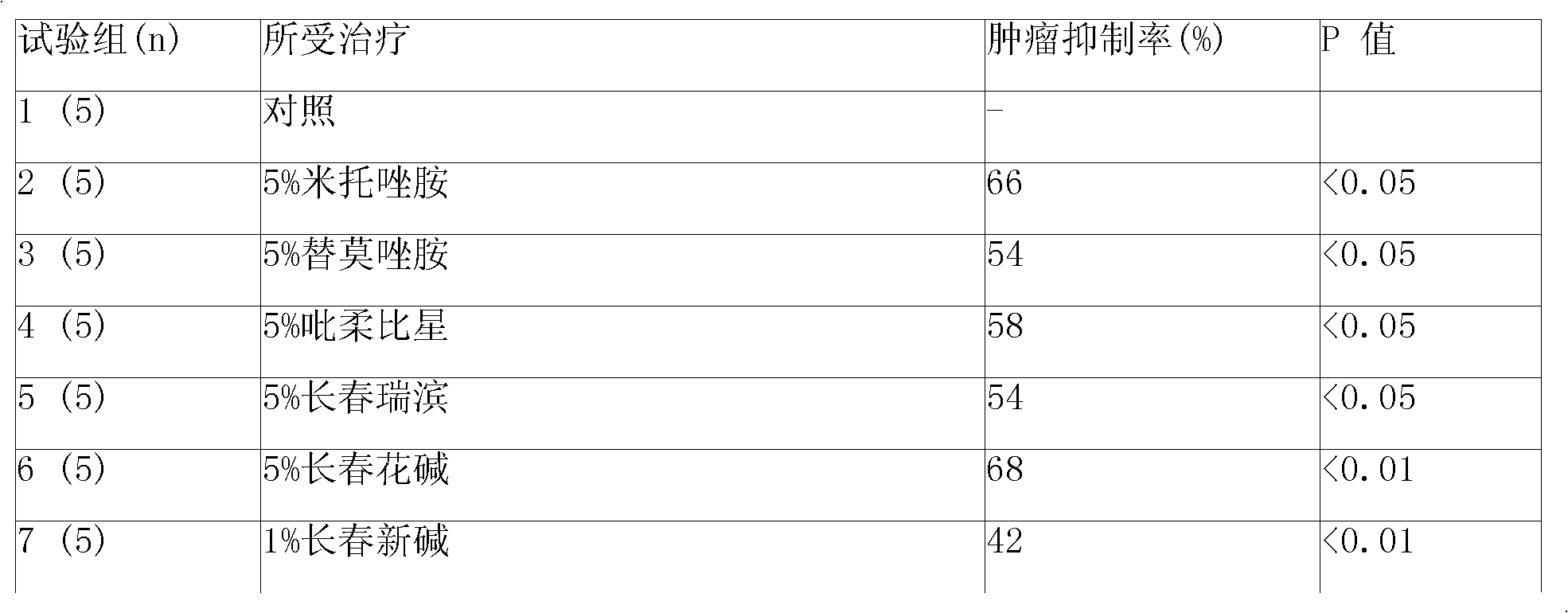
Image
Examples
Embodiment 1
[0077] Put 4, 2, 1 and 0.5g of amphiphilic block copolymers (PLGA-PEG-PLGA) into four containers of A, B, C and D respectively, and then pour them into four containers of A, B, C and D respectively Add 6, 8, 9 and 9.5 milliliters of water for injection into the container to prepare 40%, 20%, 10% and 5% hydrogels.
[0078] The molecular weight of polyethylene glycol in the amphiphilic block copolymer is 800-1200, accounting for 20% of the weight of the amphiphilic block copolymer;
[0079] In the glycolide-lactide copolymer, the molar ratio of glycolide to lactide is 6:1.
[0080] The preparation of microspheres is prepared by double emulsion method or O / W method. The auxiliary material in the sustained-release microspheres is polylactic acid / glycolic acid copolymer, wherein the blending ratio of lactic acid (LA) and glycolic acid (GA) can be 75 / 25 (W / W), the molecular weight of the copolymer of lactic acid and glycolic acid (PLGA) can be 15000-38000, and the weight ratio of ...
Embodiment 2
[0082] Measure the gelation temperature of four kinds of hydrogels in embodiment 1, the result shows that the gelation temperature of 40% and 20% hydrogel is respectively 32 ℃ (40%) and 37 ℃ (20%), and 10 The gelation temperatures of the % and 5% hydrogels were not determined at 10°C-38°C.
Embodiment 3
[0084] Put 4, 2, 1 and 0.5g of amphiphilic block copolymers (PLGA-PEG-PLGA) into four containers of A, B, C and D respectively, and then pour them into four containers of A, B, C and D respectively Add 6, 8, 9 and 9.5 milliliters of water for injection into the container to prepare 40%, 20%, 10% and 5% hydrogels.
[0085] The molecular weight of polyethylene glycol in the amphiphilic block copolymer is 1200-1600, accounting for 15% of the weight of the amphiphilic block copolymer; in the glycolide-lactide copolymer, the ratio of glycolide and lactide The molar ratio is 4:1.
[0086] The preparation of microspheres is prepared by double emulsion method or O / W method. The auxiliary material in the sustained-release microspheres is polylactic acid / glycolic acid copolymer, wherein the blending ratio of lactic acid (LA) and glycolic acid (GA) can be 75 / 25 (W / W), the molecular weight of the copolymer of lactic acid and glycolic acid (PLGA) can be 20,000-35,000, and the weight rati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com